|
APRIL 2.003
APRIL 2.003

1.) THE VIRUS OF THE SARS DIES FOR CLIMATOLOGICAL CHANGES
Author: Lapenta JJ, Dermatologist, DERMAGIC/EXPRESS
April 2.003
The first thing that we have to think with this new VIRUS of THE ATYPICAL
PNEUMONIA, of the type coronavirus who the big scientists have classified as a
MUTATION of some type of virus of THE INFLUENZA, is the places where he appeared
and the countries that I contaminate and WHEN he APPEARED. Also the mortality of
the
same one that doesn't arrive neither to 4% of the total of cases. The race but affected
the Asian.
It is said that the VIRUS APPEARED in November of the 2.002 in the China, and he
left
disseminating for different countries WHERE THE CLIMATE has allowed that the
virus
MULTIPLIES, in other countries this has not happened. As example of this can
mention to
SOUTH AMERICA where from MEXICO until the ARGENTINEAN until last week some
cases had been reported (less than 5). Neither in Venezuela, neither in
Colombia, Peru, Chile,
Paraguay, Ecuador, Mexico, Cuba, Peru, and all South America, Brazil single 2
cases with
but of 165 million inhabitants.
Already Germany development a quick method to detect the Virus, which still
continues being
disseminated, but the measures of sanitary control have avoided bigger wrongs.
In a program published by CNN in Spanish a scientist he says that to the VIRUS
you should
not combat with antibiotics BECAUSE they don't RESPOND TO THESE
MEDICATIONS, but I PARTICULARLY don't AGREE, because when administering
antibiotics and or antivirales, THESE combatting a probable secondary infection,
on the other
hand. It has been demonstrated that antibiotics as the ERYTHROMYCIN AND OTHERS
have the property of eliminating VIRAL infections, when modifying the person's
immunologic
answer.
Another aspect to stand out in my opinion is that this it is A CREATED VIRUS IN
A
LABORATORY, the genetic manipulation of the virus like BIOLOGICAL weapon is
something of what I have spoken for a long time. The VIRUS was TAKEN OUT before
the
War from IRAQ, to prove its MORTAL CAPACITY.
For years the DERMAGIC comes denouncing a POSSIBLE biological WAR, THE VIRUS
OF THE SMALLPOCK could not come out to the street, they left it kept, because
THE
ONE NUMBERS OF DIED it would reach the sky. They preferred to be played it with
a
VIRUS OF LOW MORTALITY like that of the atypical Pneumonia that has alone a
mortality of 4%. IMAGINE THE SCANDAL with A VIRUS OF HIGH MORTALITY like
that of the SMALLPOCK
I find to believe in a GENETIC MUTATION RIDICULOUS, it is a virus of
LABORATORY. Who throw it to the street? Only GOD KNOWS IT.
But those that CREATED THE VIRUS, didn't think THAT THE CLIMATOLOGICAL
CONDITIONS will put an end to THE VIRUS. Because to Suramerica I don't arrive,
and
not for SANITARY measures, I don't arrive because he doesn't HAVE CAPACITY OF
MULTIPLICATION IN OUR CLIMATE, and that will know it in Europe and those
affected countries when they change the stations. If the Virus RESISTS
undoubtedly it was
confirmed that it is SUPER a CREATED VIRUS IN A LABORATORY.
My final recommendation for ALL THOSE AFFECTED are to go up the TEMPERATURE
OF THE ROOMS WHERE THE PATIENTS are, while more hot, BETTER, more cool, more
survival for the VIRUS. Also not to forget to give antibiotics, and or
antivirals with having proven capacity to modify the patient's immunologic
answer.
greetings
Dr José Lapenta EL
VIRUS DEL SARS MUERE POR CAMBIOS CLIMATOLOGICOS
Author: Lapenta JJ, Dermatologist, DERMAGIC/EXPRESS
April 2.003 Lo primero que tenemos que
pensar con este nuevo VIRUS de LA NEUMONIA ATIPICA, del tipo coronavirus,
quienes los grandes cientificos han catalogado como una MUTACION de algun tipo
de virus de LA INFLUENZA, es los sitios donde aparecio y los paises que
contamino y CUANDO APARECIO. Tambien la mortalidad del mismo que no llega ni a
un 4% del total de casos. La raza mas afectada la Asiatica.
Se dice que el VIRUS APARECIO en Noviembre del 2.002 en la China, y se fue
diseminando por diferentes paises DONDE EL CLIMA ha permitido que el virus SE
MULTIPLIQUE, en otros paises esto no ha ocurrido. Como ejemplo de esto podemos
mencionar a SUR AMERICA donde desde MEXICO HASTA la ARGENTINA solo hasta la
semana pasada se habian reportado algunos casos (menos de 5). Ni en Venezuela,
ni en Colombia, Peru, Chile, Paraguay, Ecuador, Mexico, Cuba, Peru, y toda Sur
America, Brazil solo 2 casos con mas de 165 millones de habitantes.
Ya Alemania desarrollo un metodo rapido para detectar el Virus, el cual todavia
sigue diseminandose, pero las medidas de control sanitario han evitado males
mayores.
En un programa publicado por CNN en español un cientifico dice que al VIRUS NO
se debe combatir con antibioticos PORQUE ellos NO RESPONDEN A ESTOS
MEDICAMENTOS, pero YO PARTICULARMENTE NO ESTOY DE ACUERDO, pues al administrar
antibioticos y o antivirales, ESTAS combatiendo una probable infeccion
secundaria, por otra parte. Se ha demostrado que antibioticos como la
ERITROMICINA Y OTROS tienen la propiedad de eliminar infecciones VIRALES, al
modificar la respuesta inmunologica de la persona.
Otro aspecto a resaltar en mi opinion es que este ES UN VIRUS CREADO EN UN
LABORATORIO, la manipulacion genetica de los virus como arma BIOLOGICA ES algo
de lo que se viene hablando hace mucho tiempo. El VIRUS FUE SACADO antes de la
Guerra de IRAK, para probar su CAPACIDAD MORTAL.
Desde hace años el DERMAGIC viene
denunciando una POSIBLE GUERRA biologica, EL VIRUS DE LA VIRUELA no podia salir
a la calle, lo dejaron guardado, pues LA MORTANDAD SERIA GRANDE. Prefirieron
jugarsela con un VIRUS DE BAJA MORTALIDAD como el de la Pneumonia atipica que
tiene solo una mortalidad del 4%. Imaginense el ESCANDALO QUE ESTO HA PRODUCIDO,
que hubiese ocurrido si hubiese sido EL DE LA VIRUELA
Creer en una MUTACION GENETICA me parece RIDICULO, es un virus de LABORATORIO.
Quien lo tiro a la calle ? Solo DIOS LO SABE.
Pero los que CREARON EL VIRUS, no pensaron QUE LAS CONDICIONES CLIMATOLOGICAS
iban a acabar con EL VIRUS. Pues a Suramerica no llego, y no por medidas
SANITARIAS, no llego porque NO TIENE CAPACIDAD DE MULTIPLICACION EN NUESTRO
CLIMA, y eso lo sabremos en Europa y esos paises afectados cuando cambien las
estaciones. Si el Virus RESISTE indudablemente quedara confirmado que es un
SUPER VIRUS CREADO EN UN LABORATORIO.
Mi recomendacion final para TODOS LOS AFECTADOS es
subir la TEMPERATURA DE LAS HABITACIONES DONDE ESTAN LOS PACIENTES, mientras mas
calor MEJOR, mas frio, mas supervivencia para el VIRUS. Tambien no olvidar
suministrar antibioticos y o antivirales con probada capacidad de modificar la
respuesta inmunologica del paciente.
Saludos
Dr Jose Lapenta
2.) Smallpox Vaccination and
Cardiac Complications / Vacunacion contra viruela y complicaciones cardiacas.
By John G. Bartlett M.D.
posted 03/27/2003
Source: CDC and NYC Department of Health Alert #11 (3/26/03)
The CDC has reported 7 adverse cardiac events among 25,645 persons vaccinated
through 3/21/03. This includes 3 acute myocardial infarcts including 1 lethal
MI, 2 cases of angina and 2 cases of myopericarditis. The onset of these
complications is 2-17 days post-vaccination. The expected mortality without
vaccination based on the age and number of vaccinia recipients is 2 deaths in 3
weeks. Causal relationship is not yet established.
Myopericarditis was reported in 2 of the 25,645 health care workers who received
the vaccine, 10 of approximately 500,000 military recruits who were vaccinated,
and 1 of 17,000 Israeli health care workers (who received the Lister strain
vaccine).
As a result of these observations, the CDC is recommending that the vaccine
should be deferred in people with known cardiac disease (myocardiopathy, prior
MI, angina, or other evidence of coronary artery disease). The New York City
Health Department recommends extension of this recommendation to defer
vaccination in those with household or intimate contact with heart disease
3.) A tattooed butterfly as a vector of atypical
Mycobacteria / tatuaje en mariposa vector de una micobacteria atipica
J Am Acad Dermatol 2003;48:S73-4.
Ronni Wolf, MDa,b
Danny Wolf, MDc
Tel Aviv and Kupat Holim, Israel
Abstract
We report the first case of cutaneous inoculation of atypical Mycobacteria
secondary to tattooing. The diagnosis of atypical Mycobacteria infection of the
skin was confirmed on the basis of the clinical and histologic appearance, the
detection of acid-fast bacilli on Ziehl-Neelsen stain, and positive polymerase
chain reaction. The medical complications of tattooing, which are manifold, are
briefly summarized. This case emphasizes the need for federal regulation of
tattooing, which is an invasive procedures associated with infectious and
noninfectious complications.
4.) Thalidomide for treatment of severe
generalized discoid lupus lesions in two patients with systemic lupus
erythematosus / Talidomida para el lupus.
J Am Acad Dermatol 2003;48:S89-91.
Case Reports
Abdullah Alfadley, MD
Hanan Al Rayes, MD
Walid Hussein, MD
Abdullah Al Dalaan, MD
Khalid Al-Aboud, MD
Riyadh, Saudi Arabia
Abstract
We describe 2 patients with systemic lupus erythematous whose widespread discoid
lupus erythematosus was unresponsive to systemic steroids and antimalarial
agents. They showed dramatic improvement to thalidomide at a dose of 300 mg/d,
with maximum benefit achieved within 15 weeks of therapy. Dosages of 50 to 100
mg/d were effective in maintaining remission for 1 year. However, thalidomide-induced
neuropathy was observed in both cases.
5.) Cialis for impotency & erectile dysfunction
/ Cialis para la impotencia y disfuncion erectil
Source
http://www.cialis-impotence-drug.com/index.html
What is Cialis?
Cialis is an emerging tablet-based oral treatment for impotency & erectile
dysfunction (ED), which will soon be licensed for prescription, enabling you to
buy online. Like Levitra, Cialis is proving highly successful in clinical trials
and is generating major interest as a real alternative to Viagra and Uprima.
Cialis, being developed by Lilly, is currently undergoing approval and is
competing with Levitra to be the next major treatment for ED on the market.
Where can I get Cialis?
It is not possible to obtain genuine Cialis yet or buy Cialis online, as it is
still under its approval phase and won't be scheduled for release until early
2003. The delay in Levitra puts Cialis on equal or better competitive footing
meaning that Cialis could reach the market first.
Activemed, the UK's leading online source for safe and genuine prescription
medicines, is keeping a close eye on developments of this new impotency
treatment, and will be the first to offer this medicine online once it is
available. Check this information page for latest updates and information on
Cialis.
How effective is Cialis in treating Erectile Dysfunction?
Cialis has seen major interest worldwide following the results of a recent
clinical study on nearly 400 men with ED. The study was designed to evaluate the
efficiency of Cialis at specific time points after dosing (24 or 36 hours). The
case studies for Cialis showed that::
88 percent of men achieved erections in 30 minutes or less.
Cialis continued to stay in the system for up to 24 hours!
Cialis statically out performed the placebo at the two points of testing.
In secondary measures of efficacy - including the ability to penetrate,
satisfaction with hardness of erection and overall satisfaction, Cialis was
superior to the placebo at both 24 and 36 hours.
Does Cialis have side effects?
A small number (>5 percent) of those involved in the clinical trails reported
mild side effects with the treatment, mostly including headaches, flushing and
upset stomachs.
Cialis and cardiovascular side effects
These early tests seem to indicate that Cialis doesn't affect blood pressure as
much as other ED drugs. The clinical trials showed that statistically there was
no significant difference in cardiovascular side effects from the placebo. Tests
are still in their early stages, but Activemed will keep you informed of any
developments.
Is Cialis more effective than Viagra or Uprima?
There is currently no direct evidence from clinical trials or otherwise to
compare the effectiveness of treatments such as Cialis and Levitra, against
others such as Viagra and Uprima.
However, early findings do seem to indicate that Cialis may be especially
effective in treating ED in patients at greater risk of cardiovascular events.
Can I mix Cialis with other medicines?
This will not be known until Cialis is fully licensed and legally available.
Early tests have indicated that it should not be taken with nitrate-based
medicines for heart conditions.
Can I take Cialis if I have a heart condition?
This will not be known until Cialis is fully licensed and legally available,
although early findings do indicate that Cialis does not significantly affect
blood pressure.
6.) Papaya, the Wonder Fruit
/ La lechosa fruta maravillosa
Source:
http://www.europeanvegetarian.org/

Barbara Simonson -lecture given at the EVU Congress in Widnau 1999
from European Vegetarian, Issue 1/2000
Papayafruit
It was about 14 years ago, when I was in Maui, Hawaii, where for the first time
I had the pleasure to enjoy the sweet taste of sun-ripened, organically grown
papayas. In the health food store in Paista, I found dried papaya seeds in
little glasses, called "Papaya Enzymes", for about $5 each glass. I was
surprised. Why do people throw the seeds away when eating the fruit, and
afterwards buy them again for a lot of money? How silly! So, when my then fiancé,
Aeoliah, and I had a tropical breakfast, I ate my papaya like an apple -
including the kernels! My fiancé was shocked. "This is not the way one eats
papayas!" he shouted at me. This did not bother me, and since then I eat the
papaya whole - including skin and seeds.
Now that I have written a whole 200-page book on this "wonder fruit", the Carica
Papaya (that is its Latin name), I know why papaya seeds are so healthy, and why
you should not throw the skin away either. The people on Cuba call the papaya
"Fruta de Bomba", since it is shaped like a bomb. I discovered that the papaya
is a "bomb" of vital nutrients! It is packed with enzymes, vitamins and minerals.
The papaya-enzymes like Papain are mainly concentrated in the half green fruit
the ripe seeds as well as in the leaves of this plant. So, do not throw the
seeds away! If you do not like the bitter, spicy taste, dry them and use them
like black pepper. They look like pepper, taste like pepper, yet are much
healthier than pepper!
In Costa Rica and Mexico, the natives there call the papaya "Tree of Good Health"
and regard it as a healing remedy for almost all diseases. The papaya tree grows
very quickly, up to 10 meters in height. It doesn't need much care. In South
America, its homeland, it grows like weeds. The red Indians of South and Middle
America use the papaya not only for food, but also for healing wounds, for
supporting a weak liver, for healing constipation, against worms and parasites,
for healing inflammation and skin problems and even for treating cancer. They
are the "inventors" of modern enzyme therapy! When you live on rawfood, you can
of course forget about papaya as a healing remedy. The more sensitive you get,
the more you feel the purifying and nourishing effects of this tropical fruit,
even while eating!
What are the main ingredients of the papaya, that grows as long as 120
centimeters in Venezuela (this species is called "Lechosa")? There are a lot of
anti-oxidants in the papaya, as betacarotene- more than in carrots! - and
Vitamin C - more than in kiwis! - and a lot of Bioflavonoids. The papaya
contains a lot of minerals like Potassium, Magnesium and Calcium and is the most
alkaline fruit we know. For anybody suffering from the effects of cooked food,
sugar and meat consumption like "Acidosis" (the body gets too acid, and the
person is irritable and depressed), it is recommended to start the day with a
papaya breakfast. Some doctors recommend "papaya cures": Take at least one
papaya everyday for four weeks. After a week or so, you experience the results:
more energy, less sleep, a good mood and clear thoughts. Unbelievable?! Try it
and see for yourself!
The papaya-enzymes help to digest proteins, fats and starches. Columbus
discovered not only America, but also the papaya. When he reached the South-American
shores, he was greeted by the natives with a feast. The sailors ate too much
after the long journey, and the Indians took them to the rain-forest and offered
them papayas for releasing their pain. And it worked! So, if you overeat the
fruit Durian, which is a little hard to digest because of all the fat, you can
eat a papaya afterwards to help digestion. Of course, it is better to eat
moderately, even as a rawfoodist! The papaya-enzymes help purify your intestines
and help remove protein residue. Moreover, the papaya helps to nourish our
endocrine system, as Norman Walker has written at length in his book on juicing.
Papaya helps the body to produce more Arginin, that is an essential amino acid
that activates HGH, a growth hormone that is important for cell rejuvenation and
rebuilding of cells in the liver, in muscles and in the bones. Even the skin
benefits from Arginin - it gets smooth and is able to regenerate. The beautiful
women in the Tropics use unripe papaya for getting rid of wrinkles and old skin
cells. I tried it once, on the Canary islands - and had to scream, so much did
this hurt! Our skin is probably much thinner than the skin of these women, who
are exposed to the sun all day long. I recommend to dilute papaya juice for
treating the skin, and have included several recipes for skin masks and creams
in my papaya book.
The aborigines in Australia and the Kahunas on Hawaii use the papaya as a remedy
for cancer. The papaya should be half-ripe then, between yellow and green. I
have a friend, Halima Neumann, who was healed from stomach cancer by drinking
the juice of papayas for six months, half a liter each day, and after that
eating half-ripe papayas every day. Most of the enzymes are contained in the
fruit flesh of the unripe papaya and the seeds of the ripe one. The unripe
papaya tastes bitter, so I would not eat them normally. The unripe papaya is the
only unripe fruit that alkalines the body! Good for her: After that cure, she
decided to change her diet and become raw-foodist and started to meditate and
heal her childhood. Now, she is 50 years old, enjoys vibrant health and has
written some books in German about her experiences. She lives on the Canary
Islands and gives seminars on how to live healthily with rawfood and how to grow
organic papayas.
On Maui, there is a funny guy living in Paia who calls himself "Papaya John".
His normal name is John McCollum, and he has got a little shop called "Papaya
John", selling only papayas and papaya products like dried papayas. He regards
the papaya as medicine and is quoted in the "Maui News" with: "A Papaya a day
keeps the doctor away". Papaya John is an organic farmer and surfer and turned
papaya messiah. His mission: "My main interest is getting enzymes into you on a
daily basis.", and calls the papaya "the tropical miracle fruit".
Papaya John learnt the secrets of the papaya from Dr. Koesel, one of the
original California health food advocates, who moved to Maui in the '60s. There
the two men met, and for John, the encounter was "magic": "I knew I had some
kind of lifetime karma with this man." He studied all the health benefits of the
papaya with him. When Dr. Koesel died in 1990, one week before his 90th birthday,
for Papaya John it was obvious: "I have a commitment with God and Dr. Koesel. I
believe in the papaya and it is my task to spread the good news." He began
experiencing a level of radiant health and joy of life that he only dreamt of
before. Now he grows 22 pound papaya fruits in his garden and would not live in
a country where papayas do not grow. Papaya John smiles: "I am addicted to
papaya enzymes!" Every morning, he drinks a lot of papaya juice and feels great.
His surfboard is decorated with a huge papaya, and he takes his self-made
papaya-bars, enzyme-active, wherever he goes or travels.
You do not have to center your life on the papaya in order to benefit from it.
And, you can start trying out how much better you can feel even as a rawfoodist
by including papayas in your daily food choice! You can grow papayas easily in
your backyard garden or, if you have, in a winter-garden for colder climate
zones like Germany. In summer, I have my papaya trees outside, and take them in
in the winter time. I even composed a hymn called "A Papaya A Day" I will share
with you now. It is also included in my papaya book, including notes.
"Dismiss maya, eat papaya!" (David Wolfe)
A Papaya A Day
A papaya a day keeps the doctor away.
Take two or three and you will see:
You feel healthy and fine,
And you'll start to shine!
Life gets happy as could be,
Life gets happy as could be.
Papaya Fruit Salad
Mix pieces of ripe papaya with banana and mango slides. Serve with a little
lemon juice.
Papaya Smoothie
For this Smoothie, you need five dried apricots, a big apple, half a papaya,
pealed and cut into pieces. Soak the apricots overnight. Put everything into the
Champion and mix until smooth. Makes a great breakfast, you also can use the
Smoothie as a dressing for fruit salads.
Pina Colada
For this healthy drink, you need one cup of pineapple flesh, half a papaya,
peeled and cut into pieces, one cup of cut fresh coconut flesh, and half a cup
of pure water, if desired. Put the pineapple and papaya in the Juicer mix all
the ingredients in the mixer. Serve with a leaf of peppermint. Simply delicious!
Papaya-Salsa
You need 1 1/2 cups of papaya, cut in pieces, the same amount of sweet, red
peperoni, a teaspoon full of lemon juice, a teaspoon full of honey, very little
ground red pepper. Mix all the ingredients. This makes a great dip for a raw
food-salad.
My pocket-book "Papaya, healing with the Wonderfruit" will be available soon in
the States. (Lotus Light Publications)
BARBARA SIMONSOHN,
HOLBEINSTR. 26
D - 22607 HAMBURG
TEL.: +49 40 - 89 53 38
FAX: +49 40 - 89 34 97
E-MAIL: BASIM@BARBARA-SIMONSOHN
7.) Haemophilus aphrophilus Endocarditis after
Tongue Piercing / Endocarditis despues de piercing en la lengua.
Source;http://www.cdc.gov/
Hossein Akhondi* and Ali R. Rahimi*
*Mercer School of Medicine, Savannah, Georgia, USA
Piercing invades subcutaneous areas and has a high potential for infectious
complications. The number of case reports of endocarditis associated with
piercing is increasing. We studied a 25-year-old man with a pierced tongue, who
arrived at Memorial Health University Medical Center with fever, chills, rigors,
and shortness of breath of 6 days' duration and had an aortic valvuloplasty for
correction of congenital aortic stenosis.
Body piercing poses a risk for serious disease. Because it invades subcutaneous
areas, piercing has a high potential for infectious complications. Such
complications result from introduction of skin or mucous membrane microflora
into subcutaneous tissue or from the ongoing presence of colonies of these
microflora at the piercing site. Pain, edema, and prolonged bleeding may occur
immediately after piercing (1), and a cyst, scar, or keloid may form at the
piercing site. In various surveys, the rate of earlobe piercing infections alone
has been estimated at 11% to 24%. Skin lesions or anatomic abnormalities at the
site of piercing, as well as valvular heart disease, are risk factors for
complications (2). Staphylococcal endocarditis of the mitral valve after nasal
piercing (3), Neisseria endocarditis after tongue piercing (4), and
Staphylococcus epidermidis endocarditis and mastitis following nipple piercing
have been reported (5). Even though a consistent correlation is not known
between piercing and endocarditis, the number of case reports is increasing, and
a correlation may well exist.
Persons at high risk for complications should be treated with preventive
antibiotics, just as persons at high risk for complications receive antibiotic
treatment before dental procedures. The correlation between dental procedures
and endocarditis has been reviewed by Van der Meer et al., who prospectively
examined all cases of infective endocarditis in the Netherlands over a 2-year
period (6). Of 427 patients who had been hospitalized, 64 had previous dental or
other procedures in the preceding 3 months. Only 48 of the 438 patients met the
qualification of having native-valve and
cardiovascular anomalies that increased their risk of getting endocarditis.
Using these 48 patients as study cases, the researchers found no significant
difference in presence of dental procedures between patients and matched
controls without endocarditis (odds ratio 1.2, 95% confidence interval 0.03 to
2.3). Two other studies (7,8) reported similar results. No study has examined
the correlation between piercing and endocarditis.
In the United States, body piercing, which is becoming increasingly common, is
mainly performed by unlicenced practitioners. Only 26% of states have regulatory
authority over tattooing establishments, and only six of these states exercise
authority over body-piercing establishments. Piercing occurs in regulated and
unregulated shops, department stores, jewelry shops, homes, or physicians’
offices. Generally no antibiotic is used, and sterilization methods vary.
Studies show that ear piercing can cause cephalic tetanus (a local form of
tetanus caused by wounds or other head and neck infections) (8), Pseudomonas
infections, or perichondrial auricular abscesses, especially with Pseudomonas
aeruginosa. Tongue or oral piercing can cause Ludwig’s angina (2,9,10) or may be
complicated by normal oral flora, such as Haemophilus aphrophilus, as in this
case. Genital piercing may result in Escherichia coli infection and may increase
the risk for sexually transmitted diseases through tissue damage and exposure
and unwanted pregnancy because of condom rupture (11). Systemic infections, such
as toxic shock syndrome or sepsis, have also been reported (10). Among
noninfectious cases, granulomatous perichondritis of the nasal ala, sarcoidlike
foreign body reaction from multiple piercing, paraphimosis from a distal penis
pierce, and speech impairment, together with difficulty in chewing and
swallowing from oral jewelry, have been reported (1,2,9,10). Metal-associated
problems include allergy (especially to nickel), eczematous rash, and
lymphocytoma (2,9,10,12). We describe an incidence of H. aphrophilus
endocarditis following tongue piercing.
Case Report
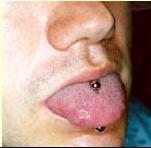
The tongue piercing of the man from this case study....
A 25-year-old man arrived at Memorial Health University Medical Center with
fever, chills, rigors, and shortness of breath of 6 days' duration. He had a
history of aortic valvuloplasty at 8 years of age for correction of congenital
aortic stenosis. At admission, the patient had fever of 38.9°C and a grade III/VI
ejection systolic murmur accompanied by a grade II/VI diastolic blowing murmur
best heard in the left sternal border area. The oral cavity was pink, and no
inflammation or exudates were noticed on the pharynx. The middle portion of the
tongue had been pierced, and a bispherical stud was in place (Figure). The
piercing was performed 2 months before onset of illness. Extensive tattoos on
the shoulders, arms, and upper torso dated back 3 years. The patient had
previous dental work done but always with antibiotic prophylaxis.
Laboratory tests showed erythrocyte sedimentation rate of 41 mm/hr (normal rate,
0–15 mm/hr) and elevated C-reactive protein of 5.1 mg/dL (normal level 0–1).
Transthoracic echocardiography was not conclusive; a transesophageal
echocardiogram showed remnants of a bicuspid and deformed aortic valve with
multiple vegetative lesions. Blood cultures were obtained, and the patient was
started on triple antibiotics (ampicillin, nafcillin, and gentamycin). Wet
preparation and acridine orange stain of the blood specimen showed gram-negative
pleomorphic rods. Two of the conventional chocolate-agar cultures turned
positive approximately 4 days after incubation and were consistent with H.
aphrophilus (β-lactamase negative, lactose fermenting, and Mannose fermenting).
The stud culture was also positive for H. aphrophilus. Antibiotics were modified
because of sensitivity to ceftriaxone and gentamycin, and the patient was
discharged to complete the 6-week course through a peripherally inserted central
catheter line at home. Aortic valve replacement was recommended after completion
of antibiotic therapy, but the patient did not return for treatment.
Conclusions
Our case demonstrates H. aphrophilus endocarditis possibly caused by tongue
piercing (or as a complication of the ongoing presence of the stud) in a patient
with congenital heart disease. Colonization around the stud likely caused
bacteremia and endocarditis. H. aphrophilus is commonly isolated from the upper
respiratory tracts of humans and animals; however, its prevalence is unknown. In
a previous study of piercing complications in patients with congenital heart
disease (13), 43% of the study population had earlobe piercing; of these, 6%
took antibiotics before piercing. Twenty-three percent of patients had piercing-related
infections 1 week to 3 years after piercing. Most infections were local skin
infections; no endocarditis was reported in that study.
Until prospective randomized studies shed light on the relationship between
piercing and endocarditis, prophylactic measures are indicated and should be
formulated, particularly for persons at high risk, e.g., those with structural
heart diseases.
Dr. Akhondi is a second-year resident with the Department of Internal Medicine
at Mercer University School of Medicine, Savannah Campus at Memorial Health
University Medical Center in Savannah, Georgia.
Dr. Rahimi is the associate director of Internal Medicine Education, chief of
the Geriatrics Division, and professor of medicine at Mercer University School
of Medicine, Savannah campus.
References
Braithwaite RL, Stephens T, Strek C, Braithwaite K. Risks associated with
tattooing and body piercing. J Public Health Policy 1999;20:459–70.
Samantha S, Tweeten M, Rickman L. Infectious complications of body piercing.
Clin Infect Dis 1998;26:735–40.
Ramage IJ, Wilson N, Thomson RB. Fashion victim: infective endocarditis after
nasal piercing. Arch Dis Child 1997;77:187.
Tronel H, Chaudemanche H, Pechier N, Doutrelant L, Hoen B. Endocarditis due to
Neisseria mucosa after tongue piercing. Clin Microbiol Infect 2001;7:275–6.
Ochsenfahrt C, Friedl R, Hannekum A, Schumacher BA. Endocarditis after nipple
piercing in a patient with a bicuspid aortic valve. Ann Thorac Surg
2001;71:1365–6.
Van der meer JT, Thompson J, Valkenburg HA, Michel MF. Epidemiology of bacterial
endocarditis in the Netherlands. II Antecedent procedures and use of prophylaxis.
Arch Intern Med 1992;152:1869–73.
Lacassin F, Hoen B, Leport C, Selton-Suty C, Delahaye F, Goulet V, et al.
Procedures associated with infective endocarditis in adults. A case control
study. Eur Heart J 1995;16:1968–74.
Strom BL, Abrutyn E, Berlin JA. Dental and cardiac risk factors for infective
endocarditis. A population-based, case-central study. Ann Intern Med
1998;129:761–9.
Koenig L, Carnes M. Body piercing, medical concerns with cutting-edge fashion. J
Gen Intern Med 1999;14:379–85.
Folz BJ, Lippert BM, Kuelkens C, Wernaer JA. Hazards of piercing and facial body
art: a report of three patients and literature review. Ann Plast Surg
2000;45:374–81
Fiumara NJ, Eisen R. The titivating penile ring. Sex Transm Dis 1983;10:43–4.
Ehrlich A, Kucenic M, Belsito DV. Role of body piercing in the induction of
metal allergies. Am J Contact Dermat 2001;12:151–5.
Cetta F, Graham LC, Lichtenberg RC. Piercing and tattooing in patients with
congenital heart diseases. J Adolesc Health 1999;24:160
8.) Is bupropion (Zyban) causing deaths? / medicina
para dejar de fumar ocasiona muertes ?
Source: http://www.mja.com.au/
Simon C Chapman and Konrad Jamrozik
The Medical Journal of 21 January 2002 176 3: 134
©The Medical Journal of Australia ISSN: Australia0025-729X3 2002www.mja.com.au
To the Editor: From 1 February to 30 June 2001, 277 602 prescriptions for the
smoking cessation drug bupropion hydrochloride (Zyban, GlaxoSmithKline) were
processed. The Health Insurance Commission approved 343 737 prescriptions for
bupropion between 1 February and 30 June.1 Comparing this figure with the 277
602 processed scripts, some 66 135 (19.2%) scripts went unfilled. One reason for
this may have been extensive publicity given to reports of deaths and numerous
adverse reactions following bupropion use.
The website of the Australian Drug Reactions Advisory Committee (ADRAC) reports
that, as at 22 June, there had been 18 reports of deaths in patients aged from
30 years to 69 years who were using or who had recently stopped using bupropion.2
ADRAC summarised intelligence on these deaths thus:
... there were a variety of reported causes of death and not a single consistent
mode of death. In addition to being smokers, several patients had other existing
risk factors for unexpected death such as alcohol abuse, diabetes or
cardiomyopathy. Eleven of the 18 patients had an alternative explanation for
death that was at least as plausible as a possible effect of bupropion. In four
reports, the available information was very limited and it was not possible to
assess the cause of death. Further information is being sought on three cases to
aid assessment of the cause of death.2
Smokers are at 3.1 times greater risk of dying (from any cause) than non-smokers
and twice as likely to die from coronary disease and stroke.3 People with
depression are three times as likely to be daily smokers4 and have double the
suicide rate of non-smokers.5
In Australia, sudden coronary fatalities occur at a rate of about 450 per
million people aged under 65,6 perhaps at a rate of 355 per million in non-smokers
and about double that in smokers. In three months (the period of recommended
bupropion use), one would expect 180 deaths per million smoker-users. Thus,
among 277 602 Australian smokers, 50 might die during any given three-month
period without any added risk from bupropion. This estimate helps to place the
18 fatalities reported to ADRAC in context.
The 277 602 scripts represent about 9.5% of Australia's 2.9 million regular
smokers. These people, their families and doctors deserve to have their
anxieties about the risks of using bupropion addressed. We would urge the
government to commission urgently a case–control study of morbidity and
mortality among smokers and their relationships to use or non-use of bupropion.
Competing interests: SC has received funding from SmithKlineBeecham (now
GlaxoSmithKline) for the preparation of professional and public educational
material on smoking in Australia.
Health Insurance Commission. Prescription data, item 8465M. 2001. <http: //www.hic.gov.au/statistics/dyn_pbs/forms/pbs_tab1.shtml#info>.
Accessed 11 Sept 2001.
ADRAC update on buproprion (Zyban). Therapeutic Goods Administration website. <http:
//www.health.gov.au/tga/docs/html/zyban.htm>. Accessed 21 Oct 2001.
Wald NJ, Hackshaw AK. Cigarette smoking: an epidemiological overview. Br Med
Bull 1996; 52: 3-11.
Breslau N, Peterson EL, Schultz LR, et al. Major depression and stages of
smoking. A longitudinal investigation. Arch Gen Psychiatry 1998; 55: 161-166.
Miller M, Hemenway D, Bell NS, et al Cigarette smoking and suicide: a
prospective study of 300,000 male active-duty Army soldiers. Am J Epidemiol
2000; 151: 1060-1063.
Beaglehole R, Stewart A, Jackson R, et al. Declining rates of coronary heart
disease in New Zealand and Australia, 1983-1993. Am J Epidemiol 1997; 145:
707-713.
Department of Public Health and Community Medicine,
9.) Polycystic Ovary Syndrome
Treatment with Insulin Lowerig Medications ./ Sindrome del
Ovario poliquistico, tratamientos con medicinas que disminuyen insulina.
Source:http
http://www.ivf.com/pcostreat.html by Mark
Perloe, M.D.
INTRODUCTION:
Polycystic ovary syndrome is characterized by anovulation (irregular or absent
menstrual periods) and hyperandrogenism (elevated serum testosterone and
androstenedione). Patients with this syndrome may complain of abnormal bleeding,
infertility, obesity, excess hair growth, hair loss and acne. In addition to the
clinical and hormonal changes associated with this condition, vaginal ultrasound
shows enlarged ovaries with an increased number of small (6-10mm) follicles
around the periphery (Polycystic Appearing Ovaries or PAO). While ultrasound
reveals that polycystic appearing ovaries are commonly seen in up to 20% of
women in the reproductive age range, PolyCystic Ovary Syndrome (PCOS) is a
estimated to affect about half as many or approximately 6-10% of women. The
condition appears to have a genetic component and those effected often have both
male and female relatives with adult-onset diabetes, obesity, elevated blood
triglycerides, high blood pressure and female relatives with infertility,
hirsutism and menstrual problems.
HYPERINSULIN & PCOS?
As of yet, we do not understand why one woman who demonstrates polycystic
appearing ovaries on ultrasound has regular menstrual cycles and no signs of
excess androgens while another develops PCOS. One of the major biochemical
features of polycystic ovary syndrome is insulin resistance accompanied by
compensatory hyperinsulinemia (elevated fasting blood insulin levels). There is
increasing data that hyperinsulinemia produces the hyperandrogenism of
polycystic ovary syndrome by increasing ovarian androgen production,
particularly testosterone and androstenedione and by decreasing the serum sex
hormone binding globulin concentration. The high levels of androgenic hormones
interfere with the pituitary ovarian axis, leading to increased LH levels,
anovulation, amenorrhea, and infertility. Hyperinsulinemia has also been
associated high blood pressure and increased clot formation and appears to be a
major risk factor for the development of heart disease, stroke and type II
diabetes.
DIAGNOSIS
There is little agreement when it comes to how PCOS is diagnosed. Most
physicians will consider this diagnosis after making sure you do not have other
conditions such as Cushing's disease (overactive adrenal gland), thyroid
problems, congenital adrenal hyperplasia or increased prolactin production by
the pituitary gland. TSH, 17-hydroxyprogesterone, prolactin and a dexamethasone
suppression test may be advisable. After reviewing your medical history, your
physicians will determine which tests are necessary. If you have irregular or
absent menstrual periods, clues from the physical exam will be considered next.
Your height and weight will be noted along with any increase facial or body hair
or loss of scalp hair, acne and acanthosis nigricans (a discoloration of the
skin under the arms, breasts and in the groin). Elevated androgen levels (male
hormones) androstenedione, DHEAS or testosterone confirm the diagnosis. A
fasting insulin and glucose level will be obtained. Many physicians tell their
patients that insulin values are normal, when in fact the value indicates that
insulin may be playing a role in stimulating the development of PCOS. Most labs
report levels less than 25-30 miu/ml as normal, while in fact, levels over
10miu/ml on a fasting blood sample suggests that PCOS may be related to
hyperinsulinism. As women with polycystic ovary syndrome may be a greater risk
for other medical conditions, testing for blood lipids, diabetes and PAI-1 (a
blood factor that promotes abnormal clotting).
NEWER METHODS OF TREATMENT
Traditional treatments have been difficult, expensive and have limited success
when used alone. Infertility treatments include weight loss diets, ovulation
medications (clomiphene, follistim, Gonal-F), ovarian drilling surgery and IVF.
Other symptoms have been managed by anti-androgen medication (birth control
pills, spironolactone, flutamide or finasteride).
Ovarian drilling can be performed at the time of laparoscopy. A laser fibre or
electrosurgical needle is used to puncture the ovary 10-12 times. This treatment
results in a dramatic lowering of male hormones within days. Studies have shown
that up to 80% will benefit from such treatment. Many who failed to ovulate with
clomiphene or metformin therapy will respond when rechallenged with these
medications after ovarian drilling. Interestingly, women in these studies who
are smokers, rarely responded to the drilling procedure. Side effects are rare,
but may result in adhesion formation or ovarian failure if the procedure is
performed by an inexperienced surgeon.
But recently promising new treatment options have become available. Drs.
Velazquez, Nestler and Dunaif have shown that lowering serum insulin
concentrations with metformin (Glucophage 1500 mg a day) or troglitazone (troglitazone,
Rezulin has recently been withdrawn from the market because of lifethreatening
side effects) ameliorates hyperandrogenism, by reduction of ovarian enzyme
activity that results in male hormone production.
For women in the reproductive age range, polycystic ovary syndrome is a serious,
common cause of infertility, because of the endocrine abnormalities which
accompany elevated insulin levels. There is increasing evidence that this
endocrine abnormality can be reversed by treatment with widely available
standard medications which are leading medicines used in this country for the
treatment of adult onset diabetes, metformin (Glucophage 500 or 850 mg three
times per day or 1000mg twice daily with meals), pioglitazone (Actos 15-30 mg
once a day), rosiglitazone (Avandia 4-8 mg once daily) or a combination of these
medications. These medications have been shown to reverse the endocrine
abnormalities seen with polycystic ovary syndrome within two or three months.
They can result in decreased hair loss, diminished facial and body hair growth,
normalization of elevated blood pressure, regulation or menses, weight loss and
normal fertility. We have seen pregnancies result in less than two months in
woman who conceived in their very first ovulatory menstrual cycle. By six months
over 90% of women treated with insulin-lowering agents will resume regular
menses.
The medical literature suggests that the endocrinopathy in most patients with
polycystic ovary syndrome can be resolved with insulin lowering therapy. This is
clinically very important because the therapy reduces hirsutism, obesity, blood
pressure, triglyceride levels, elevated blood clotting factors and facilitates
reestablishment of the normal pituitaryovarian cycle, thus often allowing
resumption of normal ovulatory cycles and pregnancy. We know the polycystic
ovary disease is associated with increased risk of heart attack and stroke
because of the associated heart attack and stroke risk factors, hypertension,
obesity, hyperandrogenism, hypertriglyceridemia, and these are to a large degree
resolved by therapy with these medications.
ARE THESE MEDICATIONS SAFE?
Side effects are rare. Although metformin, rosiglitazone and troglitazone lower
elevated blood sugar levels in diabetics, when given to nondiabetic patients,
they only lower insulin levels. Blood sugar levels will not change. In fact,
episodes of "hypoglycemic attacks" appear to be reduced.
METFORMIN (Glucophage):
When first starting this medication, people will often experience upset stomach
or diarrhea which usually resolves after the first week. This side effect can be
minimized by taking metformin with a meal and starting with a low dose. I
recommend that our patients start with one 500 mg pill daily the first week and
increase to twice a day during the second week. If after the second week GI side
effects are minimal, the dose is increased to 850 mg twice daily. Patients with
reduced renal function (creatinine >1.5 or creatinine clearance <60%) are at a
higher risk for a rare side effect of metformin therapy called lactic acidosis,
and the drug should be given cautiously, if at all, to such patients. Patients
taking metformin should notify their physician and discontinue the medication:
48 hours before surgery
48 hours before an IVP Xray study or other Xrays where an intravenous dye is
administered
If you experience shortness of breath, severe muscle weakness or chest pain
If you use alcohol excessively
TROGLITAZONE, (Rezulin) PIOGLITAZONE, (Actos), ROSIGLITAZONE, (Avandia):
These medications belong to a class of medications called PPAR gamma agonists.
They enhance the ability of smooth muscle to metabolize sugar, thereby reducing
insulin resistance.
The FDA has recently reviewed the safety of troglitazone ( and reports that 35
patients out of approximately 1.5 million have either died or required liver
transplant.) Therefore Rezulin has been removed from the market.
As the new alternatives to troglitazone, (Rezulin), Rosiglitaone (Avandia) and
pioglitazone (Actos) are metabolized by different liver enzymes experience has
shown that these medications appear to pose less risk of hepatotoxicity.
HOW DO WE MONITOR THERAPY?
BBT charts are monitored and reviewed to determine if you are ovulating. With
metformin, you will be asked to return three months after initiating therapy. If
you have ovulated, therapy may be continued another three months to see if you
will conceive. Women taking rosiglitazone or pioglitazone will be seen at two
month intervals for monitoring liver function tests (ALT). BBT charts will be
reviewed after four months. Re-evaluation will include measurements of lab tests
that were abnormal at the initial evaluation. C-peptide levels, a measure of
insulin secretion, may also be tested. If the laboratory studies are still
abnormal, metformin may be increased up to 850 mg three times daily or
rosiglitazone may be added. If the laboratory studies are normal but ovulation
has not occured, a repeat trial of clomiphene may be considered. We have seen
that women who were unable to ovulate on up to 250 mg ovulate when 50 mg of
clomiphene is used in conjunction with metformin or PPARgamma therapy.
Laparoscopic ovarian drilling may be considered for those women where other
indications for laparoscopy are present.
PREGNANCY
While safety during pregnancy has not yet been established, three patients who
continued on metformin during their entire pregnancy and one who remained on
troglitazone have delivered normal babies. There are no reports of abnormal
babies in women who conceived using metformin and all resulting babies were
normal. Metformin is a category B medication. This means that insufficient human
data is available but no credible animal data suggesting a teratogenic (could
produce birth defects) risk. Although to the best of our present knowledge the
risk of birth defects would be small, it must also be noted that maternal
diabetes has been associated with an increased risk of birth defects and the
underlying elevated insulin levels may lead to birth defects if not corrected.
While the most prudent policy may be to avoid the use of these medications
during pregnancy until more data on pregnancy outcome is available, the risk of
miscarriage may be reduced by continuing metformin during the pregnancy. We ask
our PCOS patients taking insulin-lowering medications to monitor their basal
body temperatures if pregnancy is a possibility. When the temperature remains
elevated for more than 16 days, pregnancy is likely and a home pregnancy test
should be performed. If positive, a medical consultation with the physician is
scheduled. If the EPT is negative the BBT chart is reviewed by the physician or
nurse to determine the appropriate course to follow.
MISCARRIAGE & PCOS
Women with PCOS who conceive either spontaneously or after ovulation induction
have a much higher risk of miscarriage. Liddell has shown that polycystic
appearing ovaries (on ultrasound) are more frequently seen in women with
recurrent pregnancy loss, the presence of PCO on ultrasound did not predict the
outcome in subsequent pregnancies. Hypersecretion of LH was thought to cause
chromosomally abnormal eggs leading to an increased risk of miscarriage. But a
Japanese study found that PCOS was more common in women whose prior loss was
associated with normal chromosomes. Others have suggested that high androgen
levels may be a contributory factor. Homburg has shown that miscarriage rates
after ovulation induction or IVF is decreased when women are pretreated with a
GnRH-agonist such as Synarel, Lupron or Zoladex.
Hyperinsulinemia may be a contributing factor in the higher rate of miscarriage.
Elevated levels of insulin interfere with the normal balance between factors
promoting blood clotting and those promoting breakdown of the clots. Increases
in plasminogen activator inhibitor activity (PAI-Fx) associated with high
insulin levels may result in increased blood clotting at the interface between
the uterine lining (endometrium) and the placenta. This could lead to placental
insufficiency and miscarriage.
There are no placebo-controlled clinical trials to indicate whether pregnancy
outcomes are improved in pregnancies that result from the use of insulin-lowering
medications or whether pregnancy outcomes are better in those who continue
metformin throughout the pregnancy or those who discontinue. Coetzee has shown
that use of metformin to manage non-insulin dependent diabetes during pregnancy
can be accomplished safely. We have initially noted that women who conceive
following metformin or troglitazone therapy have an unacceptably high (>30%)
risk of miscarriage. Dr. Glueck notes similar increased risk of miscarriage
following metformin therapy. He notes that the risk of miscarriage is increased
in those patients with a prior history of miscarriage, those with high LH, high
androgen levels, hyperinsulinemia or elevated PAI-Fx. Initial findings in a non-ramdomized
trial suggest a decreased risk of miscarriage if metformin is continued
throughout the pregnancy. At present there is insufficient data to routinely
advise continuation of metformin during pregnancy. As an alternative to
continuing metformin therapy, those women with increased risk of abnormal blood
clotting may benefit from baby aspirin, folate supplementation and low dose
heparin therapy. Pregnancy loss is a troubling concern. This information is
provided to enable you work with your ob/gyn physician to make an informed
decision about your care.
BIBLIOGRAPHY
1. Velazquez EM, Mendosa S, Hamer T, Sosa F, Glucck CJ. Metformin therapy in
women with polycystic ovary syndrome reduces hyperinsulinemia, insulin
resistance, hyperandrogenemia, and systolic blood pressure, while facilitating
menstrual regularity and pregnancy. Metabolism 1994,43:647655.
2. Nestler JE, Jakubowicz DJ. Decreases in ovarian cytochrome P450cl7alpha
activity and serum free testosterone after reduction of insulin secretion in
polycystic ovary syndrome. New England J Medicine 1996,335:617623.
3. Utiger RD. Insulin and the polycystic ovary syndrome. New England J Medicine
1996,335:657658
4. Dunaif A, Scott D, Finegood D, Quintana ma B, Whitcomb R. The insulin
sensitizing agent Troglitazone improves metabolic and reproductive abnormalities
in the polycystic ovary syndrome Endocrinol Metab 1996;81:32993306
5. Coetzee EJ, Jackson WP. The management of non-insulin-dependent diabets
during pregnancy. Diabetes Res Clin Pract 1985-86;1:281-287
6. Homburg R. Polycystic ovary syndrome: induction of ovulation. Ballieres
Cllinical Endocrinologys & Metabolism 1996; 10:281-292
7. Glueck CJ, Wang P, Fontaine R, Tracy T, Sieve-Smith L. Metformin-induced
resumption of normal menses in 39 of 43 (91%) previously amenorrheic women with
polycystic ovary syndrome. Metabolism 1999; 48:1-10.
8. Tulppala M, Stenman UH, Cacciatore B, Ylikorkala O. Polycystic ovaries and
levels of gonadotropins and androgens in recurrent miscarriage: preliminary
experience of 500 consecutive cases. Hum Reprod 1994;9:1328-32.
For more information on PCOS and other ovulation problems please read Miracle
Babies and Other Happy Endings Online Edition
Finasteride cream in hirsutism / Finasteride en
crema para el hirsutismo
Source:
http://www.aace.com/
ABSTRACT
Objective: To determine, in a preliminary study, whether women with hirsutism
attributable to various causes would benefit from treatment with finasteride
cream.
Methods: Finasteride cream (0.25%) and placebo cream were administered to eight
women with various degrees of facial hirsutism. The two creams were used on
opposite sides of the face in an area of excessive hair growth. The side chosen
for the finasteride cream versus placebo was randomized and blinded. In a 1 cm2
area on each side of the face, hair counts were done every 2 months throughout
the 6-month study period. Hair thickness was also measured.
Results: Hair follicles respond to testosterone by the conversion of this
androgen to dihydrotestosterone through the action of 5a-reductase. Finasteride
partially blocks this enzyme. Because of the easy solubility of this medication
through the skin, a cream applied to the area of hair growth would be expected
to decrease hirsutism locally. After a 6-month period, mean hair counts
decreased significantly from 27.5 to 15.5 (P<0.05) in the finasteride-treated
sites but showed no significant change from baseline in the placebo-applied
sites. Moreover, the mean thickness of the measured hairs (in hundredths of
millimeters) was significantly different between the placebo and finasteride-treated
sites (4.33 versus 3.11, respectively; P<0.001).
Conclusion: In this study of women with facial hirsutism, topically applied
finasteride significantly decreased hair growth and thickness, and no adverse
effects were noted. (Endocr Pract. 2001;7:5-10)
INTRODUCTION
Finasteride, a 5a-reductase inhibitor, has been extensively studied and
clinically used for treatment of prostatic hyperplasia (1,2). Currently, it is
being assessed for use in prostate cancer (3). Because both the prostate and the
hair follicle contain this enzyme, finasteride has also been used to treat male
pattern baldness (4,5). The mechanism of action of this medication is to block
the conversion of testosterone into its active metabolite dihydrotestosterone (DHT).
By inhibiting 5a-reductase, the effect of testosterone on the prostate is
blunted, and the growth of the prostatic tissue is diminished. By decreasing the
levels of DHT and not testosterone, finasteride has not been shown to affect
luteinizing hormone, follicle-stimulating hormone, or testosterone levels
substantially over time. Only 4 to 5% of men have any change in potency or
libido (6,7).
Stimulation of 5a-reductase in hair follicles causes male pattern hair to grow
on the face and body and to be lost on the scalp. In tissue culture, the hair
follicle isoenzyme was less affected by finasteride than the enzyme in the
prostate tissue culture (8).
The degree of 5a-reductase activity may account for the varied severity of
hirsutism found in women with the same levels of circulating androgens (9). Even
though the isoenzymes of 5a-reductase in the prostate (type 2) and the hair
follicles (type 1) have different structures, some cross-reactivity of
finasteride on the isoenzyme in the hair follicle may occur (10,11). The areas
of the scalp and body that are directly affected by male hormone would be the
ones most likely to be affected by inhibition of 5a-reductase.
In women, who have much lower androgen levels than do men, one might expect
finasteride to have an even greater effect on areas containing 5a-reductase. In
men with inherited 5a-reductase deficiency, facial hair is sparse, and baldness
does not develop (12,13). Women with excessive hair growth in male pattern
predominant areas may have an increased sensitivity to male hormones in the hair
follicle. Women with idiopathic hirsutism have been shown to have increased 5a-reductase
activity in their genital skin (14). Gonadotropins in women have been shown to
be unaffected by finasteride (15).
Three recent studies (16-18) have demonstrated the effectiveness of finasteride
in idiopathic hirsutism. Finasteride has also been compared with spironolactone
as a treatment for hirsutism (19,20). Because finasteride is readily soluble
through the skin (package insert, Merck & Co.), a cream containing this
medication might be beneficial therapy for mild facial hirsutism.
The current study was a preliminary investigation to determine whether women
with hirsutism attributable to various etiologic factors and with varied levels
of circulating androgens would benefit from the use of finasteride cream.
MATERIAL AND METHODS
Study Subjects
Eight consecutive patients with excessive facial hair were enrolled in the study,
including one patient with nonandrogenic facial hair. This patient was included
to ensure that the finasteride would affect only androgenic facial hair and not
have a generalized effect on all types of hair. Patients with different
severities and causes of hirsutism were included to assess whether the level of
androgen or the cause of the excessive production of androgen determined the
response to the finasteride.
Of the eight study participants, four had used low androgenic birth control
pills (Demulen in two, Tri-Cyclen in one, and Desogen in one) for more than 6
months before the start of the study. Three study subjects were taking no oral
contraceptives, and one postmenopausal patient was taking Estrace (Table 1). The
ages of the women ranged from 31 to 51 years (mean ± standard error of the mean
[SEM], 39.2 ± 2.34). The body mass index ranged from 19 to 57 kg/m2 (mean ± SEM,
36.5 ± 5.72).
Four of the subjects had polycystic ovary syndrome, two had idiopathic hirsutism,
one had hypertrichosis, and one had a mild form of congenital adrenal
hyperplasia. Polycystic ovary syndrome was defined clinically as a combination
of irregular menstrual periods, hirsutism, and obesity. Idiopathic hirsutism was
characterized by excessive hair growth in male hormone-affected areas without
concomitant increases in androgen levels or other androgenic abnormalities such
as abnormal menses, acne, or androgenic alopecia. Hypertrichosis was defined as
excessive facial and body hair on all areas of the body, not just androgen-affected
areas. Mild congenital adrenal hyperplasia was defined as an exaggerated
response of 17-hydroxyprogesterone to the intravenous administration of 250 mg
of cosyntropin.
The patients were informed about the previous uses of finasteride in the
treatment of prostate enlargement and male pattern baldness. They were told that
this medication had not been approved by the US Food and Drug Administration for
use in women and that potential side effects in women were unknown. All the
women were told that finasteride could affect a male fetus and that pregnancy
was contraindicated during the use of this medication.
Protocol
The protocol and consent form for the study were approved by the Institutional
Review Board at Northside Hospital in Atlanta, Georgia. After signing the
informed consent document, the women were interviewed to obtain a medical,
menstrual, and hirsutism history. The menstrual history included age at menarche,
regularity of menstrual cycles, age at which male pattern hair growth had begun,
previous treatment, current treatment, and whether or not male pattern baldness
was developing. A physical examination was performed; in particular, items noted
were the degree of hirsutism, body mass index, presence of acanthosis nigricans,
evidence of virilization, cushing-oid features, and male pattern baldness.
The patient's hair density was determined by counting the terminal hairs in a 1
cm2 area on either side of the patient's chin or in another area of maximal hair
growth on the face. A template was made by using landmarks of the middle of the
chin and the chin line. The hair counts on both sides were repeated every 2
months for the duration of the study. Four hairs from areas adjacent to the
template were plucked and saved for later measurement of thickness. The
thickness of the midshaft of the previously plucked hairs was blindly determined
by the use of a direct measurement microscope and measured in hundredths of
millimeters.
The pharmacokinetics of finasteride was analyzed to calculate the appropriate
concentration of the medication in the cream. Fifteen tablets of finasteride (5
mg each) were triturated (ground into a fine powder) and then wetted with 2 mL
of propylene glycol. The mixture was incorporated into Dermabase (Paddock
Laboratories, Inc.) by levigation (that is, mixed evenly by high-speed mixer and
gradually incorporated). The final cream contained 0.25% finasteride. The weight
of the tablets was 2.3 g. The weight of the Dermabase was 25.7 g. The 30-g
mixture was put into a tube. For 6 tubes, the contents were as follows: 90
tablets (weighing 13.8 g), 12 mL of propylene glycol, and 154.2 g of Dermabase.
The placebo cream consisted of the Dermabase alone in the same size and type of
tube. No difference in color or texture was evident between the placebo and the
drug-containing cream. Patients applied a thin layer of cream to the areas of
the excessive hair growth twice per day. The patients were instructed to use the
cream labeled "R" on the right side of their face and the cream labeled "L" on
the left side of the face. The women and the researcher were blinded to the
identity of the cream in each of the tubes. Randomization was accomplished by
the numbering of the cream and placebo tubes in pairs. As each woman was
enrolled in the study, she was given the next consecutive pair of numbers. The
original numbering of the tubes was done by a person not involved with the
examination of the patients or the recording of the data. The women in the study
who had been taking oral contraceptives or estrogen replacement therapy had done
so for at least 6 months before the beginning of the study. Each patient's hair
growth had stabilized before the study. Stabilization was confirmed by the
frequency of hair-removal techniques used by the study subjects. The study
participants received no other medication for hirsutism during the study and for
at least 2 months before the beginning of the study.
The finasteride cream, placebo cream, and previous doses of oral contraceptives
or estrogens were continued for the entire study. After the 6-month study period,
the patients were given the option of continuing the finasteride treatment
indefinitely or changing back to their previous medication.
The patients were seen in consultation at 2-month intervals. Questions were
asked about side effects, menstrual abnormalities, missed menstrual periods, and
changes in libido. The subjective view of the effect of the medication on the
rate of hair growth was ascertained by questioning about the number of times per
week the patients had shaved or clipped hairs. Patients' perceptions about
differences in the two sides of the face were also noted at each visit. They
were asked whether they noted any differences between the two sides and whether
they thought that they knew which cream contained the medication. Inquiries
about hair-removal techniques were made at each visit. The women had consented
to only clipping or shaving of the hairs during the study. They were told to
avoid clipping or shaving for at least 24 hours before each scheduled visit.
Electrolysis, waxing, and plucking were not permitted during the study.
Biochemical Evaluation
Women who were premenopausal had serum pregnancy tests every 2 months while the
medication was being used. The basic hirsutism evaluation (measurement of
testosterone, dehydroepiandrosterone sulfate, prolactin, and thyroid-stimulating
hormone) was completed on each patient and was repeated at the end of the 6-month
interval. Biochemical profiles and complete blood cell counts were also done at
the beginning and end of the study. All blood specimens except for the serum
pregnancy tests were submitted to commercial laboratories (National Health
Laboratory and SmithKline Laboratory). Evaluation of ovulation was not performed
for this study.
Statistical Analysis
All data are expressed as mean values ± SEM. Data from the study included hair
numbers and hair thickness for two groups: placebo versus medication (finasteride).
These data were compared with paired t tests. Significant differences were
defined as P values less than 0.05.
RESULTS
Tolerability and Safety
None of the women reported any problems with irregularity of menstrual periods,
changes in libido, changes in energy level, nausea, vomiting, diarrhea,
abdominal pain, or headache. No patient became pregnant during the study. No
study participant had any allergic reaction to the medication or skin eruption
in the areas to which the creams were applied. All serum chemistry studies and
blood cell counts remained essentially the same from the beginning until the
conclusion of the study.
Clinical Effects
By subjective evaluation, six of the eight patients noted a considerably
diminished rate of hair growth and decrease in the thickness of hairs on one
side of the face versus the other. These six women correctly guessed the side on
which the medication had been used. Five of these women continued use of the
finasteride cream after the study and have continued to show decreased facial
hirsutism.
Two study participants had no difference in their hair counts throughout the
study. One of these patients had facial hypertrichosis. This condition would not
have been expected to improve because the cause of this disorder does not
involve increased sensitivity to androgens. The other patient had been treated
with multiple combinations of medications for hirsutism during the years before
the study and had shown no major response to any of these therapies.
In comparison with baseline, hair counts in the finasteride-treated sites
decreased significantly over the duration of the study (Fig. 1). Mean (and SEM)
hair counts decreased from 27.5 ± 13.0 to 15.5 ± 7.3 (P<0.05). The placebo sites
showed no significant change in hair counts during the study. By the end of the
study, the thickness of the hairs (mean ± SEM, in hundredths of millimeters)
differed significantly between the placebo sites (4.33 ± 0.07) and the
finasteride sites (3.11 ± 0.05) (Fig. 2).
Levels of male hormones did not change significantly during the study. The
effect on the hair counts, on hair thickness, or on hair growth was not related
to the level of androgens in any patient. Likewise, blood pressure and body mass
index did not change significantly throughout the study.
DISCUSSION
Hirsutism, a devastating problem for many women, may lead to psychologic
abnormalities, including low self-esteem, withdrawal from social interaction,
isolation, and depression (10,21). Affected women may be ridiculed or pitied by
others throughout their lives. They become extremely self-conscious about their
appearance. Because the most conspicuous unwanted hair is that on the face,
women may resort to plucking, waxing, bleaching, or shaving in attempts to
eliminate this embarrassing trait. With the rapidity of hair growth seen in some
women, these hair-removing procedures achieve the desired result for only a
brief period. No drug had been approved by the US Food and Drug Administration
for this condition until recently, when eflornithine hydrochloride (Vaniqa)
cream (Bristol-Myers Squibb) was approved for inhibiting the growth of unwanted
facial hair. Spironolactone (Aldactone) (10,19,21), the most commonly used
medication for this condition, is effective but may have major side effects,
including irregular menstrual periods, gastrointestinal disturbances, fatigue,
and dizziness; it also may not result in loss of excess hair or improvement in
male pattern hair loss. Flutamide (22,23) and ketoconazole (24,25) have also
been successfully used to treat hirsutism; however, these drugs can cause
serious side effects, including liver toxicity.
The use of finasteride for treatment of hirsutism is logical because of its
specific effect on 5a-reductase, the enzyme responsible for sensitizing the hair
to testosterone. In previous studies, orally administered finasteride has been
successfully used in the treatment of hirsutism (26-28). Contraindications to
its use in women include the effect on the developing male fetus. Early
inhibition of this enzyme would be detrimental to the development of normal male
genitalia.
In this study, all the women of reproductive age were required to use some form
of contraception for the duration of the study. The only postmenopausal study
participant was receiving estrogen replacement therapy before and for the
duration of the study.
A previous study (16) assessed the use of finasteride in 17 women of
reproductive age who had idiopathic hirsutism. Only five of these women were
taking oral contraceptives; the others were using barrier methods of birth
control. Another report (19) described the use of finasteride in 14 women with
hirsutism, none of whom was taking oral contraceptives. Many medications are
contraindicated during pregnancy, and their use must be discontinued before
pregnancy. Finasteride can be used safely until a woman wants to have children.
Women using this medication must be aware of the danger to a male fetus should
they become pregnant.
The current investigation was a preliminary study designed to observe the
clinical effects of topically applied finasteride cream on facial hirsutism,
regardless of the cause of the excessive hair growth or the level of circulating
androgens. Hair thickness and hair counts showed significant improvements in the
area treated by the finasteride cream. The degree of hyperandrogenism did not
make a difference in the response of the patient to the cream. Part of the
response to the placebo cream may be attributable to the small area of the chin;
a crossover effect of the finasteride cream onto the other side of the chin may
have occurred. Thus, the separation of the two study sites may have been
suboptimal. In future studies, this problem may be eliminated by using the
placebo cream and the finasteride cream sequentially.
The long-term side effects of treatment with finasteride cream in women (except
for pregnancy concerns) are unknown; however, the specific nature of the
medication makes it unlikely to cause substantial problems. Only long-term
studies in such a setting will provide answers to this question. Nevertheless,
the use of a 0.25% topical preparation of finasteride would be expected to be
considerably safer and less expensive than the systemic medication.
CONCLUSION
Finasteride tablets and topical finasteride cream have been shown to be
effective treatments for hirsutism in women. Future studies should be undertaken
to assess long-term side effects, optimal treatment regimens, combination
therapies with spironolactone, and the effect on androgenic alopecia in women.
ACKNOWLEDGMENT
Keith Ahlfinger, PharmD, prepared the finasteride cream for this study. I
appreciate the assistance of my endocrinology colleagues in providing patients
for this study. I thank Jessica Tapia and Tabitha Sapp, who diligently organized
and helped with the details of the study, as well as the local Merck & Co.
representative for the idea for this study.
REFERENCES
Gormley GJ, Stoner E, Bruskewitz RC, et al (Finasteride Study Group). The effect
of finasteride in men with benign prostatic hyperplasia. N Engl J Med.
1992;327:1185-1191.
Stoner E (Finasteride Study Group). The clinical effects of a 5 alpha-reductase
inhibitor, finasteride, on benign prostatic hyperplasia. J Urol.
1992;147:1298-1302.
Presti JC Jr, Fair WR, Andriole G, et al. Multicenter, randomized, double-blind,
placebo controlled study to investigate the effect of finasteride (MK-906) on
stage D prostate cancer. J Urol. 1992;148:1201-1204.
Diani AR, Mulholland MJ, Shull KL, et al. Hair growth effects of oral
administration of finasteride, a steroid 5 alpha-reductase inhibitor, alone and
in combination with topical minoxidil in the balding stumptail macaque. J Clin
Endocrinol Metab. 1990;74:345-350.
Uno H, Kurata S. Chemical agents and peptide affect hair growth. J Invest
Dermatol. 1993;101(1 Suppl):143S-147S.
Rittmaster RS, Lemay A, Zwicker H, et al. Effect of finasteride, a 5 alpha-reductase
inhibitor, on serum gonadotropins in normal men. J Clin Endocrinol Metab.
1992;75:484-488.
Gormley GJ, Stoner E, Rittmaster RS, et al. Effects of finasteride (MK-906), a 5
alpha-reductase inhibitor, on circulating androgens in male volunteers. J Clin
Endocrinol Metab. 1990;70:1136-1141.
Mellin TN, Busch RD, Rasmusson GH. Azasteroids as inhibitors of testosterone 5
alpha-reductase in mammalian skin. J Steroid Biochem Mol Biol. 1993;44:121-131.
Serafini P, Lobo RA. Increased 5 alpha-reductase activity in idiopathic
hirsutism. Fertil Steril. 1985;43:74-78.
Rittmaster RS. Treating hirsutism. Endocrinologist. 1993;3:211-218.
Rittmaster RS. Finasteride. N Engl J Med. 1994;330:120-125.
Johnson L, George FW, Neaves WB, et al. Characterization of the testicular
abnormality in 5 alpha-reductase deficiency. J Clin Endocrinol Metab.
1986;63:1091-1099.
Imperato-McGinley J, Miller M, Wilson JD, Peterson RE, Shackleton C, Gajdusek
DC. A cluster of male pseudohermaphrodites with 5 alpha-reductase deficiency in
Papua New Guinea. Clin Endocrinol (Oxf). 1991;34:293-298.
Serafini P, Ablan F, Lobo RA. 5 Alpha-reductase activity in the genital skin of
hirsute women. J Clin Endocrinol Metab. 1985;60:349-355.
Fruzzetti F, de Lorenzo D, Parrini D, Ricci C. Effects of finasteride, a 5
alpha-reductase inhibitor, on circulating androgens and gonadotropin secretion
in hirsute women. J Clin Endocrinol Metab. 1994;79:831-835.
Moghetti P, Castello R, Magnani CM, et al. Clinical and hormonal effects of the
5-alpha-reductase inhibitor finasteride in idiopathic hirsutism. J Clin
Endocrinol Metab. 1994;79:1115-1121.
Castello R, Tosi F, Perrone F, Negri C, Muggeo M, Moghetti P. Outcome of long-term
treatment with the 5 alpha-reductase inhibitor finasteride in idiopathic
hirsutism: clinical and hormonal effects during a 1-year course of therapy and
1-year follow-up. Fertil Steril. 1996; 66:734-740.
Tolino A, Petrone A, Sarnacchiaro F, et al. Finasteride in the treatment of
hirsutism: new therapeutic perspectives. Fertil Steril. 1996;66:61-65.
Wong IL, Morris RS, Chang L, Spahn MA, Stanczyk FZ, Lobo RA. A prospective
randomized trial comparing finasteride to spironolactone in the treatment of
hirsute women. J Clin Endocrinol Metab. 1995;80:233-238.
Erenus M, Yucelten D, Durmusoglu F, Gurbuz O. Comparison of finasteride versus
spironolactone in the treatment of idiopathic hirsutism. Fertil Steril. 1997;68:
1000-1003.
Rittmaster RS, Loriaux DL. Hirsutism. Ann Intern Med. 1987;106:95-107.
Couzinet B, Pholsena M, Young J, Schaison G. The impact of a pure anti-androgen
(flutamide) on LH, FSH, androgens and clinical status in idiopathic hirsutism.
Clin Endocrinol (Oxf). 1993;39:157-162.
Cusan L, Dupont A, Belanger A, Tremblay RR, Manhes G, Labrie F. Treatment of
hirsutism with the pure antiandrogen flutamide. J Am Acad Dermatol. 1990;23(3 Pt
1):462-469.
Akalin S. Effects of ketoconazole in hirsute women. Acta Endocrinol (Copenh).
1991;124:19-22.
Venturoli S, Fabbri R, Dal Prato L, et al. Ketoconazole therapy for women with
acne and/or hirsutism. J Clin Endocrinol Metab. 1990;71:335-339.
Falsetti L, Gambera A, Legrenzi L, Iacobello C, Bugari G. Comparison of
finasteride versus flutamide in the treatment of hirsutism. Eur J Endocrinol.
1999;141:361-367.
Falsetti L, Gambera A. Comparison of finasteride and flutamide in the treatment
of idiopathic hirsutism. Fertil Steril. 1999;72:41-46.
Faloia E, Filipponi S, Mancini V, Di Marco S, Mantero F. Effect of finasteride
in idiopathic hirsutism. J Endocrinol Invest. 1998;21:694-698.
==================================================================
DATA-MEDICOS/DERMAGIC-EXPRESS /APRIL JOURNAL 2..003/ DR. JOSE
LAPENTA R.
===================================================================
|