| The Lyme disease,
looking for a vaccine./ La enfermedad de Lyme, buscando una vacuna. ************************************
************************************
****** DATA-MEDICOS **********
************************************
LA ENFERMEDAD DE LYME, BUSCANDO UNA VACUNA
THE LYME DISEASE, LOOKING FOR A VACCINE
**************************************
****** DERMAGIC-EXPRESS No.39 *******
****** 18 FEBERERO DE 1.999 *********
18 FEBRUARY 1.999
**************************************
***************************************
EDITORIAL ESPANOL
=================
Hola amigos DERMAGICOS, el tema de hoy LA ENFERMEDAD DE LYME, con motivo de la reciente salida al mercado de la vacuna LYMERIX, de la casa SmithKline Beecham, aprobada por la FDA el 21 de Diciembre de 1.998, y que promete ser un buen medio para detener el avance de esta patologia.
A traves de estas 24 referencias, conoceremos un poco sobre la enfermedad y algunos de los pasos que se fueron dando en inmunización hasta conseguir el producto definitivo.
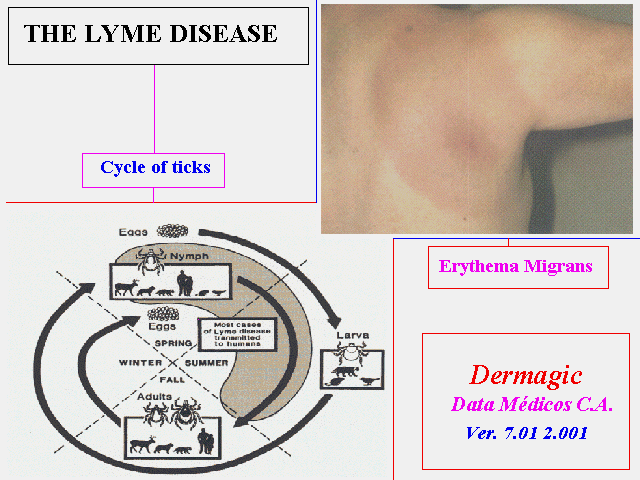
En el attach una foto CLASICA del Eritema Cronico Migrans y el ciclo evolutivo de la enfermedad.
Saludos a TODOS,,,
Dr. Jose Lapenta R.,,,
======================================================================
DERMAGIC/EXPRESS(39)
======================================================================
LA ENFERMEDAD DE LYME, BUSCANDO UNA VACUNA
THE LYME DISEASE, LOOKING FOR A VACCINE
======================================================================
1.) The Lyme Disease.
2.) Transmission-blocking immunity against Lyme disease spirochaetes associated with high levels of LA-2 equivalent antibodies.
3.) Therapeutic passive vaccination against chronic Lyme disease in mice.
4.) An ospA frame shift, identified from DNA in Lyme arthritis synovial fluid, results in an outer surface protein A that does not bind protective antibodies.
5.) Treatment-resistant Lyme arthritis may be autoimmune disease
6.) Sera from OspA-vaccinated dogs, but not those from tick-infected dogs, inhibit in vitro growth of Borrelia burgdorferi.
7.)Treatment and prevention of Lyme disease, with emphasis on antimicrobial therapy for neuroborreliosis and vaccination.
8.)Immunization with outer surface protein (Osp) A, but not OspC, provides cross-protection of mice challenged with North American isolates of Borrelia burgdorferi.
9.) Antibodies to OspB prevent infection of C3H mice challenged with Borrelia burgdorferi isolates expressing truncated OspB antigens.
10.) Recommendation to include OspA and OspB in the new immunoblotting criteria for serodiagnosis of Lyme disease.
11.) Prospects for a vaccine to prevent Lyme disease in humans.
12.) Evaluation of the safety, reactogenicity and immunogenicity of three recombinant outer surface protein (OspA) lyme vaccines in healthy adults.
13.) A vaccine consisting of recombinant Borrelia burgdorferi outer-surface protein A to prevent Lyme disease. Recombinant Outer-Surface Protein A Lyme
Disease Vaccine Study Consortium [see comments]
14.) Safety and immunogenicity of an outer surface protein A vaccine in subjects with previous Lyme disease.
15.) Vaccines against Borrelia burgdorferi have been found effective in preventing Lyme disease in two placebo-controlled trials involving more than 21 000 people from areas of the USA where Lyme disease is endemic.
16.) Reactivity with a specific epitope of outer surface protein A predicts protection from infection with the Lyme disease spirochete, Borrelia burgdorferi.
17.) Acquired resistance to Borrelia burgdorferi infection in the rabbit. Comparison between outer surface protein A vaccine- and infection-derived immunity.
18.) Incomplete protection of hamsters vaccinated with unlipidated OspA from Borrelia burgdorferi infection is associated with low levels of antibody to an epitope defined by mAb LA-2.
19.) Role of bird migration in the long-distance dispersal of Ixodes dammini, the vector of Lyme disease.
20.) FDA Committee Finds Lymerix Safe And Effective For Lyme Disease Prevention
21.) 2 vaccines found effective against Lyme disease
22.) Congratulations to SmithKline Beecham on the FDA approval of their Lyme disease vaccine! December 21, 1998
23.) First Lyme disease vaccine clears FDA
24.) Philadelphia, Pennsylvania, January 12, 1999 - LYMErix™ [Lyme Disease Vaccine
========================================================================
1.) The Lyme Disease
========================================================================
A public information guide from the Centers for Disease Control and Prevention National Center for Infectious Diseases Division of Vector-Borne Infectious Diseases
Atlanta, Georgia 30333
Lyme disease was first recognized in the United States in 1975, after a mysterious outbreak of arthritis near Lyme, Connecticut. Since then, reports of Lyme disease have increased dramatically, and the disease has become an important public health problem in some areas of the United States.
Lyme disease is an infection caused by Borrelia burgdorferi, a member of the family of spirochetes, or corkscrew-shaped bacteria.
How the disease is spread
Lyme disease is spread by the bite of ticks of the genus Ixodes that are infected with Borrelia burgdorferi. The deer (or bear) tick, which normally feeds on the white-footed mouse, the white-tailed deer, other mammals, and birds, is responsible for transmitting Lyme disease bacteria to humans in the northeastern and north-central United States. (In these regions, this tick is also responsible for the spreading of babesiosis, a disease caused by a malaria-like parasite.) On the Pacific Coast, the bacteria are transmitted to humans by the western black-legged tick, and in the southeastern states possibly by the black-legged tick.
Ixodes ticks are much smaller than common dog and cattle ticks. In their larval and nymphal stages, they are no bigger than a pinhead. Adult ticks are slightly larger.
Ticks can attach to any part of the human body but often attach to the more hidden and hairy areas such as the groin, armpits, and scalp.
Research in the eastern United States has indicated that, for the most part, ticks transmit Lyme disease to humans during the nymph stage, probably because nymphs are more likely to feed on a person and are rarely noticed because of their small size (less than 2 mm). Thus, the nymphs typically have ample time to feed and transmit the infection (ticks are most likely to transmit infection after approximately 2 or more days of feeding).
Tick larvae are smaller than the nymphs, but they rarely carry the infection at the time of feeding and are probably not important in the transmission of Lyme disease to humans.
Adult ticks can transmit the disease, but since they are larger and more likely to be removed from a person's body within a few hours, they are less likely than the nymphs to have sufficient time to transmit the infection. Moreover, adult Ixodes ticks are most active during the cooler months of the year, when outdoor activity is limited.
Ticks search for host animals from the tips of grasses and shrubs (not from trees) and transfer to animals or persons that brush against vegetation. Ticks only crawl; they do not fly or jump. Ticks found on the scalp usually have crawled there from lower parts of the body. Ticks feed on blood by inserting their mouth parts (not their whole bodies) into the skin of a host animal. They are slow feeders: a complete blood meal can take several days. As they feed, their bodies slowly enlarge.
Although in theory Lyme disease could spread through blood transfusions or other contact with infected blood or urine, no such transmission has been documented. There is no evidence that a person can get Lyme disease from the air, food or water, from sexual contact, or directly from wild or domestic animals. There is no convincing evidence that Lyme disease can be transmitted by insects such as mosquitoes, flies, or fleas.
Campers, hikers, outdoor workers, and others who frequent wooded, brushy, and grassy places are commonly exposed to ticks, and this may be important in the transmission of Lyme disease in some areas. Because new homes are often built in wooded areas, transmission of Lyme disease near homes has become an important problem in some areas of the United States. The risk of exposure to ticks is greatest in the woods and garden fringe areas of properties, but ticks may also be carried by animals into lawns and gardens.
Geographic distribution
Lyme disease has a wide distribution in northern temperate regions of the world. In the United States, the highest incidence occurs in the Northeast, from Massachusetts to Maryland. North-central states, especially Wisconsin and Minnesota. West Coast, particularly northern California.
For Lyme disease to exist in an area, at least three closely interrelated elements must be present in nature: the Lyme disease bacteria, ticks that can transmit them, and mammals (such as mice and deer) to provide food for the ticks in their various life stages. Ticks that transmit Lyme disease can be found in temperate regions that may have periods of very low or high temperature and a constant high relative humidity at ground level.
Knowing the complex life cycle of the ticks that transmit Lyme disease is important in understanding the risk of acquiring the disease and in finding ways to prevent it:
The life cycle of these ticks requires 2 years to complete. Adult ticks feed and mate on large animals, especially deer, in the fall and early spring. Female ticks then drop off these animals to lay eggs on the ground. By summer, eggs hatch into larvae.
Larvae feed on mice and other small mammals and birds in the summer and early fall and then are inactive until the next spring when they molt into nymphs.
Nymphs feed on small rodents and other small mammals and birds in the late spring and summer and molt into adults in the fall, completing the 2-year life cycle.
Larvae and nymphs typically become infected with Lyme disease bacteria when they feed on infected small animals, particularly the white-footed mouse. The bacteria remain in the tick as it changes from larva to nymph or from nymph to adult. Infected nymphs and adult ticks then bite and transmit Lyme disease bacteria to other small rodents, other animals, and humans, all in the course of their normal feeding behavior.
Lyme disease in domestic animals
Domestic animals may become infected with Lyme disease bacteria and some of these (dogs, for instance) may develop arthritis. Domestic animals can carry infected ticks into areas where humans live, but whether pet owners are more likely than others to get Lyme disease is unknown.
Symptoms and signs of Lyme disease
Early Lyme Disease: The early stage of Lyme disease is usually marked by one or more of the following symptoms and signs:
fatigue
chills and fever
headache
muscle and joint pain
swollen lymph nodes
a characteristic skin rash, called erythema migrans
Erythema migrans is a red circular patch that appears usually 3 days to 1 month after the bite of an infected tick at the site of the bite. The patch then expands, often to a large size. Sometimes many patches appear, varying in shape, depending on their location. Common sites are the thigh, groin, trunk, and the armpits. The center of the rash may clear as it enlarges, resulting in a bulls-eye appearance. The rash may be warm, but it usually is not painful. Not all rashes that occur at the site of a tick bite are due to Lyme disease, however. For example, an allergic reaction to tick saliva often occurs at the site of a tick bite. The resulting rash can be confused with the rash of Lyme disease. Allergic reactions to tick saliva usually occur within hours to a few days after the tick bite, usually do not expand, and disappear within a few days.
Late Lyme Disease: Some symptoms and signs of Lyme disease may not appear until weeks, months, or years after a tick bite:
Arthritis is most likely to appear as brief bouts of pain and swelling, usually in one or more large joints, especially the knees. Nervous system abnormalities can include numbness, pain, Bell's palsy (paralysis of the facial muscles, usually on one side), and meningitis (fever, stiff neck, and severe headache). Less frequently, irregularities of the heart rhythm occur. In some persons the rash never forms; in some, the first and only sign of Lyme disease is arthritis, and in others, nervous system problems are the only evidence of Lyme disease.
Lyme disease and pregnancy
In rare cases, Lyme disease acquired during pregnancy may lead to infection of the fetus and possibly to stillbirth, but adverse effects to the fetus have not been conclusively documented.
Diagnosis
Lyme disease is often difficult to diagnose because its symptoms and signs mimic those of many other diseases. The fever, muscle aches, and fatigue of Lyme disease can easily be mistaken for viral infections, such as influenza or infectious mononucleosis. Joint pain can be mistaken for other types of arthritis, such as rheumatoid arthritis, and neurologic signs can mimic those caused by other conditions, such as multiple sclerosis. At the same time, other types of arthritis or neurologic diseases can be misdiagnosed as Lyme disease.
Diagnosis of Lyme disease should take into account
History of possible exposure to ticks, especially in areas where Lyme disease is known to occur.
Symptoms and signs.
The results of blood tests used to determine whether the patient has antibodies to Lyme disease bacteria. These tests are most useful in later stages of illness, but even then they may give inaccurate results. Laboratory tests for Lyme disease have not yet been standardized nationally.
Treatment and prognosis
Lyme disease is treated with antibiotics under the supervision of a physician. Several antibiotics are effective. Antibiotics usually are given by mouth but may be given intravenously in more severe cases. Patients treated in the early stages with antibiotics usually recover rapidly and completely. Most patients who are treated in later stages of the disease also respond well to antibiotics. In a few patients who are treated for Lyme disease, symptoms of persisting infection may continue or recur, making additional antibiotic treatment necessary. Varying degrees of permanent damage to joints or the nervous system can develop in patients with late chronic Lyme disease. Typically these are patients in whom Lyme disease was unrecognized in the early stages or for whom the initial treatment was unsuccessful. Rare deaths from Lyme disease have been reported.
Prevention
Tick Control: Removing leaves and clearing brush and tall grass around houses and at the edges of gardens may reduce the numbers of ticks that transmit Lyme disease. This is particularly important in the eastern United States, where most transmission of Lyme disease is thought to occur near the home.
A relationship has been observed between the abundance of deer and the abundance of deer ticks in the eastern United States.
Applying acaricides (chemicals that are toxic to ticks) to gardens, lawns, and the edge of woodlands near homes is being done in some areas, but questions remain regarding its effectiveness and environmental safety. Application to residential properties should be supervised by a licensed professional pest control expert.
Reducing and managing deer populations in geographic areas where Lyme disease occurs may reduce tick abundance. Removing plants that attract deer and constructing physical barriers may help discourage deer from coming near homes.
Personal protection from tick bites
The chances of being bitten by a tick can be decreased with a few precautions.
Avoid tick-infested areas, especially in May, June, and July (many local health departments and park or extension services have information on the local distribution of ticks). Wear light-colored clothing so that ticks can be spotted more easily. Tuck pant legs into socks or boots and shirt into pants. Tape the area where pants and socks meet so that ticks cannot crawl under clothing. Spray insect repellent containing DEET on clothes and on exposed skin other than the face, or treat clothes (especially pants, socks, and shoes) with permethrin, which kills ticks on contact. Wear a hat and a long-sleeved shirt for added protection. Walk in the center of trails to avoid overhanging grass and brush. After being outdoors, remove clothing and wash and dry it at a high temperature; inspect body carefully and remove attached ticks with tweezers, grasping the tick as close to the skin surface as possible and pulling straight back with a slow steady force; aviod crushing the tick's body. In some areas, ticks (saved in a sealed container) can be submitted to the local health department for identification.
Preventive Antibiotic Treatment: Antibiotic treatment to prevent Lyme disease after a known tick bite may not be warranted. Physicians must determine whether the advantages of using antibiotics outweigh the disadvantages in any particular instance. If antibiotics are not used, physicians should alert patients to the symptoms of early Lyme disease and advise them to return for reevaluation if symptoms occur.
Lyme disease research
Research continues to discover
Where ticks are most likely to be and how best to protect against them. Which chemicals and other approaches are best for controlling ticks in each kind of habitat. Better diagnostic tests. Improved antibiotic treatment. An effective vaccine. Effects of mother's infection on the developing fetus. How Lyme disease bacteria cause chronic infections of the joints and nervous system and how to prevent these complications. For further information, contact the CDC Voice Information System at (404) 332-4555, your physician, or your local health department.
========================================================================
2.) Transmission-blocking immunity against Lyme disease spirochaetes associated with high levels of LA-2 equivalent antibodies.
========================================================================
AU: Kurtenbach-K; Dizij-A; Voet-P; Hauser-P; Simon-MM
AD: NERC Institute of Virology and Environmental Microbiology, Oxford, UK. [email protected]
SO: Vaccine. 1997 Oct; 15(15): 1670-4 ISSN: 0264-410X
PY: 1997
LA: ENGLISH
CP: ENGLAND
AB: As observed in humans, immune responses in naturally infected reservoir hosts of Borrelia burgdorferi sensu lato rarely target the outer surface proteins (Osp) A and B of Lyme disease spirochaetes. The absence of protective immunity in such hosts following tick-borne infection allows them to play an effective role in the maintenance of Lyme borreliosis in nature. Therefore, the question was addressed whether one of the most prominent natural reservoir host species of B. burgdorferi s.l. in Europe, the yellow-necked mouse (Apodemus flavicollis), may lack the ability to elicit transmission-blocking antibodies to Lyme borreliosis spirochaetes. Yellow-necked mice were immunized with a recombinant lipidated OspA from B. burgdorferi sensu stricto or with high numbers of UV-irradiated whole spirochaetes. All immunized mice, but not untreated controls, developed polyclonal humoral immune responses to OspA (31 kDa). Serum antibodies of animals vaccinated with the recombinant OspA contained high levels of antibody to an epitope of OspA, defined by the monoclonal antibody LA-2, whereas only low levels of LA-2 equivalent antibodies could be detected in sera from animals immunized with killed spirochaetes. Ixodes ricinus ticks infected with B. burgdorferi s.s. lost their spirochaete load after feeding on animals with high levels of LA-2 equivalent antibody; ticks feeding on animals which had only low or undetectable serum levels of LA-2 equivalent antibodies retained their spirochaete infection. Furthermore, animals with high levels of LA-2 equivalent antibody were protected against spirochaete infection. Our study shows that natural mouse reservoir hosts are highly competent to generate transmission-blocking antibodies after vaccination with a lipidated recombinant OspA and indicates that antibodies to the LA-2 epitope play a key role in the destruction of B. burgdorferi s.s. within feeding Ixodes ricinus ticks.
========================================================================
3.) Therapeutic passive vaccination against chronic Lyme disease in mice.
========================================================================
AU: Zhong-W; Stehle-T; Museteanu-C; Siebers-A; Gern-L; Kramer-M; Wallich-R; Simon-MM
AD: Max-Planck-Institut fur Immunbiologie, Stubeweg 51, D-79108 Freiburg, Germany.
SO: Proc-Natl-Acad-Sci-U-S-A. 1997 Nov 11; 94(23): 12533-8 ISSN: 0027-8424
PY: 1997
LA: ENGLISH
CP: UNITED-STATES
AB: Passive and active immunization against outer surface protein A (OspA) has been successful in protecting laboratory animals against subsequent infection with Borrelia burgdorferi. Antibodies (Abs) to OspA convey full protection, but only when they are present at the time of infection. Abs inactivate spirochetes within the tick and block their transmission to mammals, but do not affect established infection because of the loss of OspA in the vertebrate host. Our initial finding that the presence of high serum titers of anti-OspC Abs (5 to 10 &mgr;g/ml) correlates with spontaneous resolution of disease and infection in experimentally challenged immunocompetent mice suggested that therapeutic vaccination with OspC may be feasible. We now show that polyclonal and monospecific mouse immune sera to recombinant OspC, but not to OspA, of B. burgdorferi resolve chronic arthritis and carditis and clear disseminated spirochetes in experimentally infected C.B.-17 severe combined immunodeficient mice in a dose-dependent manner. This was verified by macroscopical and microscopical examination of affected tissues and recultivation of spirochetes from ear biopsies. Complete resolution of disease and infection was achieved, independent of whether OspC-specific immune sera (10 microg OspC-specific Abs) were repeatedly given (4x in 3- to 4-day intervals) before the onset (day 10 postinfection) or at the time of fully established arthritis and carditis (days 19 or 60 postinfection). The results indicate that in mice spirochetes constitutively express OspC and are readily susceptible to protective OspC-specific Abs throughout the infection. Thus, an OspC-based vaccine appears to be a candidate for therapy of Lyme disease.
========================================================================
4.) An ospA frame shift, identified from DNA in Lyme arthritis synovial fluid, results in an outer surface protein A that does not bind protective antibodies.
========================================================================
AU: Fikrig-E; Liu-B; Fu-LL; Das-S; Smallwood-JI; Flavell-RA; Persing-DH;
Schoen-RT; Barthold-SW; Malawista-SE AD: Department of Internal Medicine, Yale University School of Medicine, New Haven, CT 06520-8031, USA.
SO: J-Immunol. 1995 Dec 15; 155(12): 5700-4 ISSN: 0022-1767
PY: 1995
LA: ENGLISH
CP: UNITED-STATES
AB: Passive immunization with murine or human Abs to outer surface protein A (OspA) can protect mice against Borrelia burgdorferi, but OspA Abs elicited during natural infection in mice or humans are unable to clear the spirochete from the infected host. To examine Ab binding by OspA during the course of human infection, we amplified the operon encoding full-length ospA and ospB from synovial fluids of a patient with chronic Lyme arthritis, the first such recoveries from human material, at four separate time points over 4.5 mo, and expressed OspA in Escherichia coli. OspA mAbs that passively protected mice from infection did not bind one of the expressed OspAs, because of a deletion in ospA that resulted in a frame shift and premature stop codon near the carboxyl terminus. However, expressed OspA from a later synovial fluid sample did not contain this deletion. Thus, although altered forms of OspA, which potentially can influence host immune effectiveness, do occur in the human host, they cannot be the only factors responsible for microbial persistence.
========================================================================
5.) Treatment-resistant Lyme arthritis may be autoimmune disease
========================================================================
Source: The Lancet
Antibiotic treatment of Lyme disease is usually effective. But a small proportion of patients develop arthritis that persists for months or years despite eradication of the causative agent, Borrelia burgdorferi. Now, evidence suggests that this treatment-resistant Lyme arthritis is an autoimmune disease, a finding that could have implications for vaccine safety.
Patients with treatment-resistant Lyme arthritis have an increased frequency of the HLA allele DRB1*0401, which is also associated with rheumatoid arthritis. So, Dawn Gross (Tufts University, Boston, MA, USA) and US colleagues hypothesised that this allele might bind part of the B burgdorferi outer-surface lipoprotein A (OspA). By in-vitro and animal studies, the team identifed the specific immunodominant OspA epitope. A gene-bank search then revealed a human protein--leukocyte-function-associated antigen 1 (LFA-1)--which has sequence homology to the OspA epitope and so could act as an autoantigen. T cells from patients with treatment-resistant Lyme arthritis, but not those from patients with other chronic arthritides, reacted to whole OspA, the immunodominant OspA epitope, and LFA-1. The authors propose that an immune reaction to B burgdorferi may upregulate LFA-1, and start a "vicious cycle", in which T cells continue to react to LFA-1 even after the spirochaete has been eliminated (Science 1998; 281: 703-06).
Though not discussed by the authors, these findings might also raise questions over safety of immunisation against Lyme disease. It seems theoretically possible that OspA vaccines could prompt autoimmunity in susceptible individuals, though no such reaction has been observed in large trials.
Kelly Morris
========================================================================
========================================================================
6.) Sera from OspA-vaccinated dogs, but not those from tick-infected dogs, inhibit in vitro growth of Borrelia burgdorferi.
========================================================================
AU: Straubinger-RK; Chang-YF; Jacobson-RH; Appel-MJ
AD: James A. Baker Institute for Animal Health, College of Veterinary Medicine, Cornell University, Ithaca, New York 14853, USA.
SO: J-Clin-Microbiol. 1995 Oct; 33(10): 2745-51 ISSN: 0095-1137
PY: 1995
LA: ENGLISH
CP: UNITED-STATES
AB: Dogs were challenged with Borrelia burgdorferi by exposure to ticks, with or without prior protection from infection by recombinant OspA (rOspA) vaccination. Sera from these dogs were tested for their capability to inhibit the growth of B. burgdorferi in vitro. Bacterial growth was detected by a color change in the culture medium, and the optical density was measured with a spectrophotometer in microtiter plates. By growth inhibition, which was complement dependent, the color change was lacking after 5 days of incubation. Over a 1-year study, nonvaccinated dogs infected by exposure to ticks showed high antibody titers to B. burgdorferi by kinetic enzyme-linked immunosorbent assay (KELA). The same sera did not inhibit spirochetal growth or did so only at a low dilution. These results corresponded to the lack of OspA and OspB antibodies seen in Western blots (immunoblots), and these dogs were not protected from infection or disease. In contrast, dogs immunized with rOspA prior to challenge with infected ticks produced high antibody titers, as determined by KELA, but their sera also had high growth-inhibiting antibody titers. Western blot analysis showed a strong band in the 32-kDa region when the sera of these dogs were tested. When adjuvant was administered with rOspA, antibody titers by both KELA and growth inhibition were higher and persisted longer in the immunized dogs. All dogs immunized with rOspA were protected from infection and disease.
========================================================================
7.)Treatment and prevention of Lyme disease, with emphasis on antimicrobial therapy for neuroborreliosis and vaccination.
========================================================================
Wormser GP
Division of Infectious Diseases, New York Medical College, Valhalla, USA.
Semin Neurol (UNITED STATES) Mar 1997 17 (1) p45-52 ISSN: 0271-8235
Language: ENGLISH
Document Type: JOURNAL ARTICLE; REVIEW; REVIEW, TUTORIAL
Journal Announcement: 9709
Subfile: INDEX MEDICUS
Antibiotic therapy is recommended for all forms of neuroborreliosis. Although stage 2 neuroborreliosis will usually resolve without any treatment, antibiotic therapy has been associated with faster resolution of symptoms and may prevent additional non-neurologic disease manifestations. Ceftriaxone is the most convenient parenteral agent for stage 2 and 3 neuroborreliosis because of its once-daily dosage. Available data indicate that a 2-4-week treatment course is adequate for most patients. Patients with isolated seventh nerve palsy may be treated with an oral agent (for example, doxycycline). Recombinant outer surface protein A of Borrelia burgdorferi is a highly protective immunogen for prevention of Lyme disease in experimental animals. Humoral immunity is sufficient for protection. A recombinant OspA vaccine has been licensed for prevention of Lyme disease in dogs. Licensure of an OspA vaccine for humans will depend on a critical analysis of the results of recently completed efficacy studies. (91 References)
========================================================================
8.) Immunization with outer surface protein (Osp) A, but not OspC, provides cross-protection of mice challenged with North American isolates of Borrelia burgdorferi.
========================================================================
Probert WS; Crawford M; Cadiz RB; LeFebvre RB
Department of Pathology, Microbiology, and Immunology, School of Veterinary Medicine, University of California, Davis 95616, USA.
J Infect Dis (UNITED STATES) Feb 1997 175 (2) p400-5 ISSN: 0022-1899
Language: ENGLISH
Document Type: CLINICAL TRIAL; JOURNAL ARTICLE
Journal Announcement: 9709
Subfile: AIM; INDEX MEDICUS
The identification of antigens with the capacity to induce a broad spectrum of protective immunity is an important consideration in the design of a Lyme disease vaccine. In this study, the range of protection provided by outer surface protein (Osp) A or OspC vaccination was compared. Mice actively immunized with OspA or OspC were challenged with 3 North American isolates of Borrelia burgdorferi. OspA-immunized mice were fully protected from infection with each of the isolates, whereas mice immunized with OspC were protected from infection with the homologous isolate but not with 2 heterologous isolates. Sequence analysis revealed that the ospA genes from these 3 isolates were >99% homologous, whereas the ospC genes shared only 81%-85% homology. Western blot analysis suggested antigenic heterogeneity associated with OspC but not OspA. These results indicate that genetic and antigenic heterogeneity may limit the usefulness of OspC as a vaccine constituent.
========================================================================
9.) Antibodies to OspB prevent infection of C3H mice challenged with Borrelia burgdorferi isolates expressing truncated OspB antigens.
========================================================================
Probert WS; Crawford M; LeFebvre RB
Department of Pathology, Microbiology, and Immunology School of Veterinary Medicine University of California, Davis 95616, USA.
Vaccine (ENGLAND) Jan 1997 15 (1) p15-9 ISSN: 0264-410X
Language: ENGLISH
Document Type: JOURNAL ARTICLE
Journal Announcement: 9708
Subfile: INDEX MEDICUS
Truncation of outer surface protein B (OspB) of the Lyme disease agent, Borrelia burgdorferi, may allow the organism to escape immunological destruction and render an OspB-based vaccine ineffective. To investigate this possibility, we have identified two isolates, 297 and CA4, which predominantly express a truncated form of the OspB antigen. In each case, nucleic acid sequencing revealed that truncation of the OspB antigen resulted from a nonsense mutation within the 3', end of the ospB gene. Growth inhibition and protection studies demonstrated that both isolates were neutralized by an anti-OspB serum. Our results indicate that truncated forms of the OspB antigen possess epitopes that may represent important targets for neutralizing antibodies and thus, support the inclusion of OspB as a component of a subunit vaccine.
========================================================================
10.) Recommendation to include OspA and OspB in the new immunoblotting criteria for serodiagnosis of Lyme disease.
========================================================================
Author Hilton E; Devoti J; Sood S
Address Department of Medicine, Long Island Jewish Medical Center, New Hyde Park, New York 11042, USA.
Source J Clin Microbiol, 34(6):1353-4 1996 Jun
Abstract
In October 1994, the Second National Conference on the Serologic Diagnosis of Lyme Disease recommended a two-step approach to serological testing. The first step was the performance of an enzyme-linked immunosorbent assay (ELISA); the second step was a confirmatory immunoblot. New criteria for the interpretation of a positive immunoblot were also recommended. The committee decided to omit the 31- and 34-kDa bands (OspA and OspB, respectively) from the choice of bands considered diagnostic for a positive immunoblot. Since we had previously included these in our diagnostic criteria for Lyme disease-positive immunoblots, we reviewed data for all patients attending a Lyme disease center with positive ELISAs and immunoblot assays for Lyme disease from 1 September 1992 to 31 December 1993. The criteria for a positive Western blot (immunoblot) were the presence of 5 or 12 bands, including the 10 recommended by the conference, and the presence of the 31- and 34-kDa protein bands. Of the 136 patients evaluated, 50 were considered to have Lyme disease. Of these 50, 4 (8%) would not have met immunoblot criteria for the diagnosis if the new recommendations were used. Had the 31- and 34-kDa bands been included as part of the diagnostic requirements for immunoblot, these patients would have been included. Although overdiagnosis of Lyme disease appears to be the more frequent problem, our concern is that the exclusion of the 31- and 34-kDa protein bands from the diagnostic criteria may result in the underdiagnosis of Lyme disease by those who would rely too heavily on serological confirmation. The addition of the 31- and 34-kDa bands to those recommended for confirmatory immunoblot should be reconsidered.
========================================================================
11.) Prospects for a vaccine to prevent Lyme disease in humans.
========================================================================
AU: Wormser-GP
AD: Division of Infectious Diseases, Westchester County Medical Center, Valhalla, New York 10595, USA.
SO: Clin-Infect-Dis. 1995 Nov; 21(5): 1267-74
ISSN: 1058-4838 PY: 1995
LA: ENGLISH
CP: UNITED-STATES
AB: Vaccination with recombinant outer-surface protein A (OspA) preparations has been highly successful in protecting laboratory animals against challenge by strains of Borrelia burgdorferi closely related to the one from which the OspA was derived. Humoral immunity is sufficient for protection. Against natural infection introduced by ticks, the vaccine-induced immune response may begin to take effect in the tick itself--i.e., before the spirochete enters the host--and may extend to a broader spectrum of strains of B. burgdorferi than are represented in the vaccine. Single recombinant OspA vaccine preparations are currently being evaluated in two large-scale efficacy trials in adults in the United States. Greater heterogeneity among B. burgdorferi strains in Europe than among those in the United States will likely necessitate the development of a vaccine containing antigens from multiple strains; a multivalent vaccine may or may not be needed in the United States.
========================================================================
12.) Evaluation of the safety, reactogenicity and immunogenicity of three recombinant outer surface protein (OspA) lyme vaccines in healthy adults.
========================================================================
Van Hoecke C; Comberbach M; De Grave D; Desmons P; Fu D; Hauser P; Lebacq E; Lobet Y; Voet P
SmithKline Beecham Biologicals, Rixensart, Belgium.
Vaccine (ENGLAND) Dec 1996 14 (17-18) p1620-6 ISSN: 0264-410X
Language: ENGLISH
Document Type: CLINICAL TRIAL; JOURNAL ARTICLE; RANDOMIZED CONTROLLED TRIAL
Journal Announcement: 9707
Subfile: INDEX MEDICUS
The safety, reactogenicity and immunogenicity of three candidate Lyme vaccines based on recombinant outer surface protein (OspA) presented in either lipidated or unlipidated forms, were assessed in 300 seronegative volunteers. Subjects received three doses of one of the three formulations at monthly intervals and were evaluated for antibody levels and the presence of symptoms after each dose. All formulations proved to be safe, the majority of local reactions being reported as mild, and all general symptoms were perceived to be either-mild or moderate in intensity. No subject refused a subsequent vaccine dose. All subjects were tested for both anti-OspA IgG and LA-2 equivalent antibodies up until day 84. All three vaccines induced an immune response but subjects who received lipoprotein OspA had the highest anti-OspA IgG and LA-2 equivalent GMTs after each dose and this was also true for the subset of subjects tested on day 180. The lipoprotein OspA group also had the largest number of subjects who remained seropositive for anti-OspA IgG antibodies. As the lipoprotein formulation produced the strongest immune response, with symptoms which were acceptable to all the vaccinees, we suggest further development of this vaccine.
========================================================================
13.) A vaccine consisting of recombinant Borrelia burgdorferi outer-surface protein A to prevent Lyme disease. Recombinant Outer-Surface Protein A Lyme Disease Vaccine Study Consortium [see comments]
========================================================================
Author Sigal LH; Zahradnik JM; Lavin P; Patella SJ; Bryant G; Haselby R; Hilton E; Kunkel M; Adler-Klein D; Doherty T; Evans J; Malawista SE
Address Department of Medicine, University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School, New Brunswick 08903-0019, USA.
Source N Engl J Med, 339(4):216-22 1998 Jul 23
Abstract
BACKGROUND: Lyme disease is a multisystem inflammatory disease caused by infection with the tick-borne spirochete Borrelia burgdorferi and is the most common vector-borne infection in the United States. We assessed the efficacy of a recombinant vaccine consisting of outer-surface protein A (OspA) without adjuvant in subjects at risk for Lyme disease.
METHODS: For this double-blind trial, 10,305 subjects 18 years of age or older were recruited at 14 sites in areas of the United States where Lyme disease was endemic; the subjects were randomly assigned to receive either placebo (5149 subjects) or 30 microg of OspA vaccine (5156 subjects). The first two injections were administered 1 month apart, and 7515 subjects also received a booster dose at 12 months. The subjects were observed for two seasons during which the risk of transmission of Lyme disease was high. The primary end point was the number of new clinically and serologically confirmed cases of Lyme disease.
RESULTS: The efficacy of the vaccine was 68 percent in the first year of the study in the entire population and 92 percent in the second year among the 3745 subjects who received the third injection. The vaccine was well tolerated. There was a higher incidence of mild, self-limited local and systemic reactions in the vaccine group, but only during the seven days after vaccination. There was no significant increase in the frequency of arthritis or neurologic events in vaccine recipients.
CONCLUSIONS: In this study, OspA vaccine was safe and effective in the prevention of Lyme disease.
========================================================================
14.) Safety and immunogenicity of an outer surface protein A vaccine in subjects with previous Lyme disease.
========================================================================
AU: Schoen-RT; Meurice-F; Brunet-CM; Cretella-S; Krause-DS; Craft-JE; Fikrig-E
AD: Department of Internal Medicine, Yale University School of Medicine, New Haven, Connecticut, USA.
SO: J-Infect-Dis. 1995 Nov; 172(5): 1324-9 ISSN: 0022-1899
PY: 1995
LA: ENGLISH
CP: UNITED-STATES
AB: The safety and immunogenicity of a recombinant outer surface protein A (OspA) Lyme vaccine in patients previously diagnosed with Lyme disease was assessed in a dose-ranging, prospective study. Thirty healthy volunteers were consecutively assigned to receive three doses of 3, 10, or 30 micrograms of OspA vaccine at 0, 1, and 2 months. Subjects were seen 3 days after each vaccine dose and 1 month after completion of the three-dose schedule. Local side effects included soreness, induration, swelling, and redness. Transient systemic side effects occurred in 21 subjects, the majority of which (81%) were characterized as mild. Solicited symptoms included migratory mild arthralgias that lasted 24 h in 3 subjects. Sideeffects were not more evident after the second or third dose. Of the patients, 93% developed high-titer OspA antibodies. Thus, an OspA vaccine may be safe and immunogenic in patients with a history of Lyme disease.
========================================================================
15.) Vaccines against Borrelia burgdorferi have been found effective in preventing Lyme disease in two placebo-controlled trials involving more than 21 000 people from areas of the USA where Lyme disease is endemic.
========================================================================
Source: The Lancet
Lyme disease, spread by ticks, is the commonest vectorborne disease in the USA, with about 10 000 new cases being reported to the Centers for Disease Control and Prevention (Atlanta, GA) each year. The vaccines tested consist of recombinant B burgdorferi outer-surface lipoprotein A (OspA). In both trials, two doses of vaccine were given a month apart, with a booster dose at 12 months.
In one trial, headed by Leonard Sigal (University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School, New Brunswick, NJ, USA), vaccine efficacy--defined as no signs or symptoms of Lyme disease and no IgG or IgM antibodies to B burgdorferi detected on western blotting--was 68% in the first year of the study, after two injections, and 92% in the second year, after the booster dose. In the other trial, led by Allen Steere (Tufts University School of Medicine, Boston, MA, USA), the efficacy of vaccine plus adjuvant--defined as no signs or symptoms of Lyme disease--was 49% in the first year and 76% in the second year. Both vaccines were well tolerated, the only ill-effects being mild or moderate local and systemic reactions lasting a few days after vaccination (N Engl J Med 1998; 339: 209-15 , 216-22 ).
Arthritis and neuroborreliosis, which can be prevented with antibiotics, can develop in symptom-free infected people. Data on IgG seroconversions from Steere et al show that their vaccine was 83% effective in preventing symptomless Lyme disease in the first year and 100% effective in the second year.
Sigal et al suggest that concerns about the efficacy of a vaccine that contains only a single OspA protein may be unjustified. Their vaccine "prevented Lyme disease at locations in five different states in the United States, suggesting that it provides coverage against a wide range of strains". However, they add, no inference can be drawn about the ability of this vaccine to protect against the European species B garinii and B afzelii.
In an editorial , Roy Steigbigel and Jorge Benach (State University of New York at Stony Brook, NY, USA) say "an effective, safe, and affordable vaccine for people who live in areas where Lyme disease is endemic would be welcome" because measures to prevent exposure to ticks, such as use of insect repellents and tucking one's trousers into one's socks before venturing into areas where ticks are common, are "bothersome and of unknown effectiveness". They note that the need for and frequency of booster immunisation must be investigated, since the duration of protective immunity is unknown and high antibody titres will be required to eliminate the spirochaete. "Now that the B burgdorferi genome has been sequenced, itis likely that even more effective vaccines will become available."
Dorothy Bonn
========================================================================
16.) Reactivity with a specific epitope of outer surface protein A predicts protection from infection with the Lyme disease spirochete, Borrelia burgdorferi.
========================================================================
Golde WT; Piesman J; Dolan MC; Kramer M; Hauser P; Lobet Y; Capiau C;
Desmons P; Voet P; Dearwester D; Frantz JC
National Center for Infectious Diseases, Centers for Disease Control and Prevention, Public Health Service, U.S. Department of Health and Human Services, Fort Collins, Colorado 80522, USA.
Infect Immun (UNITED STATES) Mar 1997 65 (3) p882-9 ISSN: 0019-9567
Language: ENGLISH
Document Type: JOURNAL ARTICLE
Journal Announcement: 9705
Subfile: INDEX MEDICUS
The response to recombinant vaccines for Lyme disease was studied to determine serum antibody levels effective in protecting against tick- transmitted infection. Data presented here demonstrate a significant correlation between antibody to an epitope on outer surface protein A (OspA) and protection against infection with Borrelia burgdorferi in canines and mice. A competitive enzyme-linked immunosorbent assay was developed to measure antibody to a site on OspA, defined by monoclonal antibody LA-2. Comparison of LA-2 titers against infection of canines and mice following vaccination and challenge established a predicted value for LA-2 titers. The statistical relationship between serum antibody levels and protection was calculated by logistic regression analysis. The statistical model predicted that an LA-2 titer of 0.32 microg equivalents (eq) per ml correlated to an 80% predicted probability of protection for both mice and dogs. This value was used to classify mice and dogs as to their protected status at the time of tick exposure. The LA-2 cutoff titer (0.32 microg eq/ml) correctly classified all dogs (n = 13) and mice (n = 44) that failed to become infected. By contrast, 20 of 22 dogs and 28 of 31 mice with titers of less than 0.32 microg eq/ml became infected. On the basis of these results, we conclude that an LA-2 titer is a reliable indicator of immune status for estimating immune protection following use of OspA-based vaccines for B. burgdorferi sensu stricto.
========================================================================
17.) Acquired resistance to Borrelia burgdorferi infection in the rabbit. Comparison between outer surface protein A vaccine- and infection-derived immunity.
========================================================================
Foley DM; Wang YP; Wu XY; Blanco DR; Lovett MA; Miller JN
Department of Microbiology and Immunology, University of California, Los Angeles, School of Medicine, 90024, USA.
J Clin Invest (UNITED STATES) Apr 15 1997 99 (8) p2030-5 ISSN: 0021- 9738 Contract/Grant No.: AI-37312--AI--NIAID; AI-29733--AI--NIAID; 2-T32- AI-07323--AI--NIAID
Language: ENGLISH
Document Type: JOURNAL ARTICLE
Journal Announcement: 9707
Subfile: AIM; INDEX MEDICUS
Intradermal inoculation of the rabbit with Borrelia burgdorferi, sensu lato, results in the consistent development of erythema migrans (EM), dermal infection, and visceral dissemination of the spirochete. Within 5 mo, EM as well as dermal and visceral infection are cleared and the animals exhibit immunity to reinfection. This study compares infection- derived immunity with acquired resistance resulting from the administration of a lipidated recombinant outer surface protein A (OspA) vaccine presently undergoing human trial. 4 of 11 OspA vaccinated rabbits, challenged intradermally at each of 10 sites with 10(5) low passage B. burgdorferi, developed EM as well as dermal and disseminated infection. After identical challenge, 2 of the 11 infection-immune rabbits developed a dermal infection, but not EM or disseminated infection. Further, ELISA anti-OspA titers did not correlate with the status of immunity for either OspA vaccinated or infection-immune rabbits. Prechallenge ELISA anti-OspA titers were relatively low in the infection- immune group. This study demonstrates that a state of partial immunity to experimental Lyme disease may result that could potentially mask infection. Further, our data strongly suggest that immunogen(s) other than OspA is/are responsible for stimulating acquired resistance in the infection- immune rabbit.
========================================================================
18.) Incomplete protection of hamsters vaccinated with unlipidated OspA from Borrelia burgdorferi infection is associated with low levels of antibody to an epitope defined by mAb LA-2.
========================================================================
AU: Johnson-BJ; Sviat-SL; Happ-CM; Dunn-JJ; Frantz-JC; Mayer-LW; Piesman-J
AD: Division of Vector-Borne Infectious Disease, Centers for Disease Control and Prevention (CDC), Fort Collins, CO 80522, USA.
SO: Vaccine. 1995 Aug; 13(12): 1086-94 ISSN: 0264-410X
PY: 1995
LA: ENGLISH
CP: ENGLAND
AB: Efforts to develop a recombinant vaccine for Lyme disease have focused on using the outer surface protein A (OspA) of Borrelia burgdorferi as an immunogen. We evaluated the effectiveness of an unlipidated recombinant OspA as a vaccine in hamsters. This molecule is soluble and can be produced in high yield in Escherichia coli, characteristics that permit simple and relatively low cost production. Vaccination with unlipidated OspA protected a substantial portion of animals--59-79%, depending on the challenge strain and route--against moderate doses of spirochetes delivered either by injection or by bite of infected nymphal ticks (Ixodes scapularis). The instances of vaccine failure were associated with development of low levels of antibody to a particular OspA epitope, one defined by mAb LA-2. At least 50 ng ml-1 of LA-2 equivalent antibody was necessary for protection of hamsters. Lower LA-2 equivalent antibody concentrations occurred in unprotected animals in the presence of high-titered polyclonal antibody to native OspA. A competitive binding assay to quantitate this serum fraction is described that should be of use in monitoring the quality of the antibody response to OspA in vaccine trials. Concentrations of LA-2 equivalent antibody parallel the ability of the serum specimens to inhibit the growth of B. burgdorferi in culture.
========================================================================
19.) Role of bird migration in the long-distance dispersal of Ixodes dammini, the vector of Lyme disease.
========================================================================
AU: Smith-RP Jr; Rand-PW; Lacombe-EH; Morris-SR; Holmes-DW; Caporale-DA
AD: Maine Medical Center Research Institute, South Portland 04106-3295, USA.
SO: J-Infect-Dis. 1996 Jul; 174(1): 221-4
ISSN: 0022-1899
PY: 1996
LA: ENGLISH
CP: UNITED-STATES
AB: To evaluate the role of migratory birds in the long-distance dispersal of Ixodes dammini ticks and in the spread of Lyme disease, a 6-year study of migrating birds to an offshore New England island was conducted during 1989-1994. I. dammini are not endemic on this island, therefore allowing assessment of long-distance tick dispersal rather than local infestation. Of 11,324 spring migrants examined, 1.2% were infested with I. dammini. Of 8607 fall migrants examined, 0.2% were infested. Of nymphal ticks examined, 20% were infected with Borrelia burgdorferi. OspB DNA sequencing of 6 B. burgdorferi isolates was identical to sequences of 2 strains common in coastal Maine. It is evident that bird migration allows for long-distance dispersal of I. dammini from areas where they are endemic to areas where they are not and that a few bird species account for the majority of tick dispersal. The likelihood of establishment of enzootic Lyme disease by this mechanism is discussed.
========================================================================
20.) FDA Committee Finds Lymerix Safe And Effective For Lyme Disease Prevention
========================================================================
PHILADELPHIA, PA -- May 27, 1998 -- SmithKline Beecham’s Lymerix(TM) [Lyme Disease Vaccine (Recombinant OspA)] was found safe and effective for the prevention of Lyme disease by the United States Food and Drug Administration’s vaccine and related biological products advisory committee.
The committee's recommendation, though not binding, will be considered by the FDA in its final review of the Product License Application.
Lymerix is under review at the FDA for the prevention of both definite Lyme disease (characteristic symptoms with laboratory confirmation) and asymptomatic infection (no immediate physical symptoms, but laboratory confirmation of infection). Asymptomatic infection with bacterium causing Lyme disease can be especially problematic since an individual who does not exhibit early signs of the disease can still develop serious late-stage, debilitating symptoms months to years later, including arthritic or neurologic conditions.
Lyme disease has rapidly become the most common tick-borne illness and one of the fastest-growing infectious diseases in the U.S., with cases now reported to the Centers for Disease Control and Prevention from 48 states.
People at special risk include those who live or work in endemic areas in the U.S., including New England, the mid-Atlantic states, the upper Midwest and the Pacific Northwest.
Lyme disease is caused by a bacterium, Borrelia burgdorferi, which is carried by ticks that transmit the infection from animals (including deer and rodents) to humans. Since approximately 15 to 30 percent of Lyme disease cases are asymptomatic in the period immediately following infection, such individuals may progress to late-stage Lyme disease. The bacteria can affect the joints, tendons, heart or nervous system, resulting in arthritis, heart abnormalities such as heart block and myocarditis (inflammation of the muscular walls of heart) and Bell's palsy (paralysis of one or both sides of the face).
A SB-sponsored clinical trial enrolled more than 10,000 individuals ranging from 15 to 70 years of age at 31 U.S. sites. The study participants received three injections of either Lymerix or placebo. The study results demonstrated that this immunisation series produced efficacy rates of 79 percent against definite Lyme disease and 100 percent against asymptomatic infection. The most frequently reported adverse event was reaction at the injection site.
========================================================================
21.) 2 vaccines found effective against Lyme disease
========================================================================
By The Associated Press
BOSTON -- Two newly developed vaccines against Lyme disease are probably equally effective, researchers say. Final reports on large-scale testing of the competing products were published in today's issue of the New England Journal of Medicine. The findings were first made public last September at a medical conference in San Francisco.
The two vaccines are similar but not identical. They are LYMErix, produced by SmithKline Beecham, and ImuLyme, made by Pasteur Merieux Connaught.
Neither vaccine is on the market yet. In May, a Food and Drug
Administration advisory board recommended sale of LYMErix. It has not looked at ImuLyme yet. Both vaccines require three shots and take a year to reach full effectiveness. In the second year, LYMErix was found to be 76 percent protective against Lyme disease, and ImuLyme was 92 percent effective. However, Dr. Leonard H. Sigal, who directed the ImuLyme study, noted that the volunteer groups who tested the two vaccines were not identical and this could have accounted for the difference in outcome. "I don't think one can say one vaccine is better than the other," said Sigal, a researcher at New Jersey's Robert Wood Johnson Medical School in New Brunswick. "It may be they are equally efficacious." In the studies, LYMErix was tested on 10,936 people and ImuLyme on 10,305. Both were tried in parts of the country where Lyme disease is common, including Connecticut. The disease is named after Lyme, Conn., where it was first recognized.
========================================================================
22.) Congratulations to SmithKline Beecham on the FDA approval of their Lyme disease vaccine! December 21, 1998
========================================================================
Lyme Disease Vaccine: Hope or Hype?
Hartford, CT - As the news about two vaccines is being released, we need to ask if this is hope for a real solution to a public health crisis or hype to grab onto the 100-200 million dollar Jackpot?
The Lyme Disease Foundation is very optimistic about the upcoming announcements of the results of the three -year vaccine trials. "We have always believed that the development of a Lyme disease vaccine was the best solution to the growing epidemic, even before any manufacturer decided to develop one. But, as the trial results are presented to the general public, we must have answers to the two most fundamental questions. Is it safe? Does it protect people from Lyme disease?" Says Karen Vanderhoof-Forschner, MBA, CPCU, CLU, Chair, Board of Directors of the Lyme Disease Foundation. Are the results being fully discussed in a full scientific presentation in front of Lyme disease knowledgeable professionals?
Failure to ask the tough questions, as the media places this news into headlines, can result in both false hope and people discarding vital prevention strategies. The result of false hope could mean increased cases of Lyme disease, other tick-borne disorders, and additional human suffering. Vaccine manufacturers will welcome discussion of these questions.
The announcements are being released in two separate poster abstracts at the 1997 Infectious Disease Society Association's 1997 meeting. Both vaccines are recombinants of an outer surface protein (Osp) A of the Lyme bacterium - Borrelia burgdorferi, which was discovered by LDF Board member Willy Burgdorfer, PhD, MD (hon).
So far over 102,000 cases of confirmed Lyme disease have been reported to the government. This represents the tip of the iceberg in terms of the total cases of Lyme disease - which could be closer to 2 million. If the vaccine is a success it could stem the rising cost of Lyme disease to Society (which is about $1 billion) and reduce the personal and professional devastations that have been occurring to families across the United States.
The following are concerns already expressed within the scientific community and general public:
1. Is it safe?
It appears to be safe when given to healthy people who do not have Lyme disease. But the LDF has been cautioned by scientists concerned that theyhave not yet discovered what all the pieces of OspA do. Is this important? It may be. Think of OspA as a large molecule, having 100 - 1,000 subpieces. Some of those pieces may cause a high antibody response. Others may cause a protective response. Yet, others may cause damage, which may take years to be detected.
2. Is OspA protective?
The vaccine is immunogenic, meaning it does cause a strong antibody response - but is this response protective? Questions have been raised by some published studies. Padilla et al. (1996-University of Wisconsin School of Medicine), conducted borreliacidal antibody research on the bloods of patients who had the vaccine and in hamsters. This test measures productive antibody response. The conclusion was that high levels of antibody were produced, the protective portion was short-lived, with many people not having protection by 180 days after the second injection. Leaving some people unprotected from infection. Another finding has been that high antibody levels did not correlate to the vaccine's effectiveness. Therefore, blood tests could not be used as criteria for success or failure. Finally the research found that the protective response did protect against various strains for a very limited time. And, that later, the protection was limit to the strain used in the vaccine's development. There are about 100 strains in the US. A 1997 study by Foley et al (UCLA School of Medicine) also found that the vaccine antibody test results did not correlate with the status of immunity. And, that a state of partial immunity can occur where the subject could have infection harbored in the skin, yet have neither a rash nor overt evidence of infection. This may result in "partial immunity " that could "potentially mask infection." This potentially means that after several years of vaccines, the patient may decide to forgo further vaccinations, resulting in the bacteria leaving the skin and establishing disease. Further, their data strongly suggested that another immuogen(s) was responsible for the protective immune response.
3. What are the trials designs?
What are the criteria for success and failure? Reliance on the governments case reporting criteria does miss many cases of real disease. And, the CDC does state that their definition is not for clinical use - and how much more clinical could something get? How were the patients followed? Did all patients receive in-person physical or yearly blood tests/evaluations? What monitoring of the patients was done? By mail? In person - was blood drawn and were "silent converters" caught early. These are the people who are infected but not yet symptomatic.
4. Do the Principle Investigators (PI) believe a vaccine is needed?
Both principle investigators have stated that Lyme disease is easy to diagnose and treat. And, that Lyme disease is over diagnosed and overtreated. Then, why are they conducting trials on a product that may not be needed?
5. This is both Good News and Bad News
The good news is that the vaccines have the real potential to work and help stem a public health crisis. These may indeed work! The Bad News is that it may take several years of scientific presentations and debate before the FDA approves these for widespread public use. And, once released, the vaccines may be restricted to a government bureaucrats view of who should be allowed to have it, and not be open to all who want it. If these vaccines work, then this will be the second most important scientific advancement in the 100 year history of Lyme disease.
SmithKline Beecham Pasteur Merieux Connaught
Number of people: 10,906 entering 10,306 entering Drop-out rate: 9% 25% Centers: Multiple centers in endemic areas 14 centers in endemic areas Ages: 15-70 18-92 Injection Schedule: 3 injections - 0, 1 mo., 12 mo. 3 injections - 0, 1 mo., 12 mo. Biochemical: Recombinant adjuvanted Bb outer surface protein (Osp) A. Recombinant Bb outer surface protein (Osp) A. Monitoring: Suspected LD cultured, PCR, serologic test. Blood tests at beginning (0), 12 mo., 20 mo. Blood drawn at rash (unclear if any other tests were done). Group: People with positive blood tests excluded. People with Lyme disease excluded. LD patients included. Efficacy: ¾ 65: 3 doses 90% Overall: 2 doses 50%. 3 doses 79%. (If people w/asymptomatic infection "silent converters" were incl. as successes, then protection was 82% after dose 2 and 100% after dose 3. But, these people were counted as treatment failures.) ¾ 59: 2 doses 82%. 3 doses 100% No testing for antibody conversion, without specific limited signs and symptoms. "Silent converters" missed. Treatment Failure: Failures were those whose blood tests went from negative to positive. Unclear what other criteria for failures were used. Failures must have a positive blood test and meet the CDC LD Case reporting definition. EM rash alone may not have qualified. Special Note: „ 66 less protection. Mild to moderate local & general self-limited side effects. „ 60: No efficacy after 2 doses. 75% after 3rd dose. "generally well tolerated."
This comparison is made using the available information.
1 Schutzer SE, Coyle PK. (1997) Detection of Lyme disease after OspA vaccine. The New England Journal of Medicine. 337. Letter to the editor - September 11.
2 Padilla ML, Callister SM, et al. (1996) Characterization of the protective borreliacidal antibody response in humans and hamsters after vaccination with a Borrelia burgdorferi outer surface protein A. Journal of Infectious Diseases. 174:139-46.
3 Foley DM, Wang Y-P, et al. (1997) Acquired resistance to Borrelia burgdorferi infection in the rabbit. Journal of Clinical Investigation. 99:2030-2035.
========================================================================
23.) First Lyme disease vaccine clears FDA
========================================================================
By LAURAN NEERGAARD
WASHINGTON (December 21, 1998 4:49 p.m. EST http://www.nandotimes.com) - The Food and Drug Administration OK'd the world's first vaccine against Lyme disease Monday, a shot anxiously awaited in tick-infested regions - but that won't eliminate the threat.
Doctors warned that although SmithKline Beecham's LYMErix will help prevent Lyme disease, it isn't 100 percent protective and it takes three shots over a full year to build optimal immunity. So people still must take precautions against ticks, the FDA stressed.
"You'll have to continue to use insecticides, continue to check for and remove attached ticks," said FDA immunologist Karen Elkins. As one researcher put it, she added, "People should not consider this a license to run willy-nilly through the woods."
The FDA approved LYMErix for vaccination of people ages 15 to 70 who live or work in grassy or wooded areas where Lyme disease-bearing ticks are present. Although doctors say a Lyme vaccine is urgently needed for children, SmithKline is still studying whether LYMErix is safe in children and adequately protects them.
Another big question is how long the protection offered by those first three LYMErix shots will last. SmithKline is studying whether people will need yearly booster shots.
SmithKline said it will ship the vaccine to doctors' offices in a few weeks, but refused to disclose the price.
Lyme disease is caused by a bacterium carried by pin-sized ticks that live in wooded and grassy areas nationwide, but especially in the Northeast and upper Midwest. The government counted about 16,000 new cases in 1996, an increasing number and one that critics say the government seriously undercounts.
Typically, Lyme disease causes a telltale bull's-eye rash plus fatigue, chills, fevers and joint pain. Antibiotics can cure it. But if it's left untreated, Lyme disease can severely damage the heart and nervous system.
Until now, doctors' best advice was to use insecticide, watch for ticks and, when venturing into tick-prone areas like unmowed grass or brush, wear long sleeves and pants tucked into socks or boots.
But that's hard advice to follow since ticks are most active in the summer.
In addition, the tiny ticks are hard to spot and some patients don't suffer early Lyme symptoms, so not everyone at risk knows to seek help.
SmithKline's LYMErix offers the first immune protection. The vaccine creates antibodies that recognize an outer protein of the Lyme bacterium, called Osp-A.
Unlike typical vaccines, LYMErix can fight the bacterium while the germ is still inside the tick, before the victim becomes infected. When the tick sucks a vaccinated person's blood, it will ingest the antibodies, which can neutralize Lyme germs inside the tick.
In a study of almost 11,000 people in high-Lyme states, volunteers received three shots: Two given a month apart anywhere from January to April, just before the Northeast's peak tick season, and a third shot a year later.
During that first summer tick season, when patients had received only two LYMErix shots, the vaccine proved 50 percent protective.
But after the third shot, during the following summer, patients who got LYMErix had 78 percent fewer cases of Lyme disease than people who got three dummy shots.
That's a complicated vaccine schedule - and one that requires consumers to understand that even if they start getting LYMErix this winter or spring, they won't have optimal protection until summer 2000, the government said.
SmithKline now is studying whether people could get equal effectiveness if the three shots were given over a shorter time period, and hopes to give the FDA that data in about a month.
LYMErix's main side effects were soreness and swelling at the injection site and occasional muscle and joint pain, the FDA said.
And the vaccine cannot be used by everyone.
The FDA said LYMErix should not be taken by pregnant women, people with such joint problems as rheumatoid arthritis, or people with chronic illnesses related to prior Lyme infections. SmithKline has not studied the vaccine's safety in those people.
========================================================================
24.) Philadelphia, Pennsylvania, January 12, 1999 - LYMErix™ [Lyme DiseaseVaccine
========================================================================
(Recombinant OspA)], the world’s first vaccine to prevent Lyme disease, isnow available. Manufactured by SmithKline Beecham Biologicals (NYSE: SBH), LYMErix™ was proven safe and effective in the prevention of both definite Lyme disease (characteristic symptoms with serologic and culture diagnosis) and asymptomatic infection (no symptoms, but serologic diagnosis). LYMErix™ received approval from the U.S. Food and Drug Administration in December, 1998.
"People should talk to their doctors about getting vaccinated with LYMErix™ now in order to begin building immunity for the upcoming Lyme season," Said Michael Caldwell, MD., clinical trial investigator and commissioner of the Dutchess County Department of Health in Poughkeepsie, New York.
LYMErix™ is a genetically engineered vaccine that contains lipoprotein OspA, an outer surface protein of the causative bacterium, Borrelia burgdorferi. A novel hypothesis has been proposed to explain the effectiveness of lipoprotein OspA vaccination: when infected ticks bite humans who have been vaccinated with LYMErix™, the vaccine-induced antibodies are taken up by the tick and interact with the Borrelia burgdorferi in the midgut of the tick, thereby preventing transmission of the organism to the host.
In a landmark clinical trial with 10,936 individuals ranging from 15 to 70 years of age at 31 U.S. sites in endemic areas, LYMErix™ demonstrated vaccine efficacy rates of 78 percent against definite Lyme disease and 100 percent against asymptomatic infection after a complete immunization series (three vaccine doses given on a 0, 1 and 12 month schedule). Trials have been completed with vaccination on accelerated dosing schedules and SmithKline Beecham expects to file with the FDA shortly.
LYMErix™ is available in 30 mcg/0.5mL vials and Tip-Lok™ syringes. Each 0.5 mL of vaccine consists of 30 mcg of lipoprotein OspA adsorbed on 0.5 mg aluminum as aluminum hydroxide. LYMErix™ is indicated for individuals 15 to 70 years of age. LYMErix™ may be associated with local injection-site reactions including redness and swelling, flu-like symptoms, arthralgias and myalgias. As with any vaccine, LYMErix™ may not protect 100 percent of individuals.
"SmithKline Beecham is proud to be the first company in the world to offer safe and effective protection to millions of Americans at risk for this potentially debilitating and often misdiagnosed disease," said Eddie Gray, vice president and director, SmithKline Beecham’s U.S. Vaccine Business Unit.
Lyme Disease: A Widespread and Potentially Debilitating Disease
Lyme disease is a potentially serious multi-stage bacterial infection with a wide range of symptoms—from a characteristic skin rash and flu-like symptoms to arthritis and heart abnormalities. It has rapidly become the most common tick-borne illness, with cases reported in 48 U.S. states. Over 99,000 cases have been reported to the Centers for Disease Control and Prevention from 1982 to 1996. People at highest risk include those living in, working in or planning to travel to endemic areas in the U.S., including the Northeast, upper Midwest and Pacific coastal areas.
Lyme disease is caused by a bacterium, Borrelia burgdorferi, which is carried by ticks that transmit the infection from animals to humans. In the pivotal efficacy trial also published in The New England Journal of Medicine, approximately 20 percent of people infected with the Borrelia burgdorferi organism did not exhibit any symptoms (asymptomatic infection). Such infection, if undiagnosed and left untreated, can progress to late-stage Lyme disease. Those infected may develop debilitating symptoms months to years after including arthritic or neurologic problems possibly requiring extensive treatment.
Source: SmithKline Beecham
=====================================================================
DATA-MEDICOS/DERMAGIC-EXPRESS No (39) 16/02/99 DR. JOSE LAPENTA R.
======================================================================
|