Cleaning and Polishing Brass (II)
Oxidation | Cleaning | Polishing | Ammonia | CyanideEffects of Quality | Stress Corrosion Cracking
Commercial Products | Electrolysis
Effects of Quality:
When impurities in metals are found in higher concentrations insome areas and in lower concentrations in other areas, thedifference in electrical potential between the metal atoms and theatoms of the impurities would promote oxidation whenever anelectrolyte (salt bridge) is present on the surface of the metal,forming tiny voltaic cells on the surface. These cells can work inseries and accelerate the oxidation process even more. If theimpurities could be distributed perfectly evenly in the metals, theuniform distribution would minimize the collective effect (voltaiccells working in series) and thereby minimize the rate of oxidation.Because of considerable improvements in the production methodsof metals, modern metals (particularly since WWII) are much morehomogenous (the impurities are more evenly distributed in themetals) and have fewer impurities. Modern metals will oxidize lessquickly than metals made by older production methods. Thedifferences can be seen in antique and modern clocks.
Production methods in antique clocks frequently includingpounding, beating, and hammering of the brass to the desired shapeand thickness, resulting in hardening of the brass and in stresses.The stresses tend to cause stress cracking in the metal on amicroscopic level at the points of highest stress and of greatestweakness in the metal structure. Very serious stress cracking inpoorly fabricated metal can sometimes be visible to the eye. Themicroscopic stress cracking is revealed when the oxide layer isremoved. Note that the use of ammonia or even of acid for a few minutes to removethe oxide layer does not cause stress cracking. The use of thesechemicals merely reveals pre-existing defects in the metals. Sincethe amount of metal removed is very small indeed (in brass,perhaps one tenth of one percent), the harm done by the removal isinsignificant. A purist, however, would not want to do this to avaluable antique timepiece!
A modern manufacturing technique used to evenly distribute the stresses in brass clockplates can be seen on many Hermle clocks, which have a pattern stamped onto the plates.Brass obtained from large rolls of sheet metal has a tendency to return to its curved shape,giving the plates a tendency to warp: the pattern that is stamped onto the plates stretchesthe brass and removes the tendency to warp so that the plates remain flat. Combine thistechnique with modern methods of manufacturing brass, resulting in much lower levelsof impurities that are much more evenly distributed throughout the metal, and the endresult is a product of extremely consistent quality that the manufacturer can control veryprecisely.
Tempered steel that has an unequal distribution of temper (in otherwords, that was not tempered evenly) and that has an unevendistribution of impurities is similarly vulnerable to stress cracking,which is usually what causes mainsprings to break.
Stress Corrosion Cracking:
A much more serious form of stress cracking could occur if the brass were exposed to anoxidizing agent or an acidic environment with the presence of ammonia or ammoniumsalts, because they form complex ions with zinc and copper, namely tetraaminezinc [Zn(NH3)4]++ and tetraaminecopper [Cu(NH3)4(H2O)2]++ ions (which gives the acqueous solution a blue colour).
The protective layers of zinc and copper oxides, which protect the metal underneathbecause they are insoluble in water, become soluble with the formation of the complexions and are removed from the surface of the metal, exposing fresh metal to be oxidized.The oxidation continues unimpeded until the metal dissolves. If a stress is applied to thebrass (such as hanging a weight from a brass hook), the brass will crack at the points ofgreatest stress when enough metal is oxidized to cause the lattice structure of the metal tofail. For example, if you hang a lamp from a brass hook in a barn (where there is plentyof moist air, carbon dioxide and ammonia produced by the animals), the moist air resultsin the formation of a layer of electrolyte (weak carbonic acid from carbon dioxidedissolved in water) and the right conditions are created for corrosion to take place,unimpeded by any protective layers of insoluble oxides or hydroxides as the ammonia makes themetal ions soluble. The warmth that rises from the lamp below accelerates the corrosion,the hook cracks and breaks, allowing the lamp to fall. The cracking that takes place hereis called "Stress Corrosion Cracking" because this cracking is a direct result of corrosion.This cracking takes place when a stress is applied, but not if a stress is not applied: thebrass hook will not crack if the lamp is not hanging from it! The brass hook will corrode,though.
Note, however, that the clock cleaning solution does not have any acidity(hydrogen ions in solution) nor any oxidizing agents because the solution is alkaline andammonium hydroxide is a reducing agent. Since metals do not oxidize in alkalinesolutions, the ammonia present does not dissolve the metal atoms underneath the layer ofmetal ions that form the metal oxides. The layer of oxide is removed and bare metal isexposed, but no corrosion takes place in the solution. No corrosion takes place until themetal is removed from the solution and exposed to air. There are no applied stresseseither (unless you submerge the entire clock movement with the mainsprings wound up: anyone who does this should promptly depart from this profession anyway) since the clock should bedisassembled prior to cleaning. The necessary conditions are not met when the brass issubmerged in ammoniated clock cleaning solution for stress corrosion cracking to takeplace, so stress corrosion cracking does not happen here. In the equations above, the zinc and copper oxides become tetraaminezinc acetate andtetraaminecopper acetate.
Commercial Products:
Commercially-prepared ammoniated solutions for cleaning clocksare more sophisticated. The traditional formula includesammonium hydroxide solution, oleic acid and acetone. The acetoneis a powerful organic solvent that is also soluble in water becausethe oxygen atom is polarized and forms a weak bond with thehydrogen atoms of water molecules. As an organic solvent, acetoneaids in the removal of dirt and old oils from the clock parts and indissolving the oleic acid. The oleic acid is made from maize (corn)and is basically an alkene with a long carbon chain and acarboxylic acid group at one end.
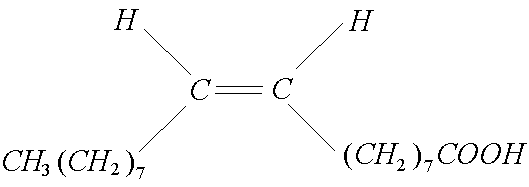 |
which could be represented as: R-COOH
Oleic acid is a liquid at room temperature (at least in Texas)because it is an unsaturated fatty acid. (For comparison, stearicacid, which also has a carbon chain with eighteen carbon atoms, isa saturated fatty acid, so it has a higher melting point. Stearic acidis made from animal and vegetable fats.) Oleic acid is a weak acidlike acetic acid because it ionizes very little in aqueous solution,but it does not dissolve in water the way acetic acid does, forminginstead a suspension of groups of organic ions called Micelles,thereby behaving like a soap. Oleic acid reacts with ammoniumhydroxide to produce ammonium soap:
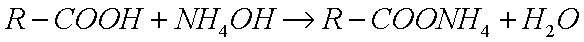 |
This reacts with metal oxides to produce metallic soaps:
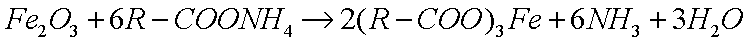 |
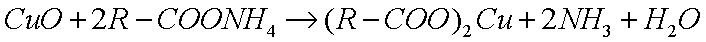 |
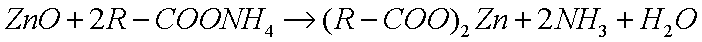 |
The oleic acid therefore has two functions: to remove the oxidelayer from the metals and to act as a soap to remove the dirt andoils. The cleaning solution has about 90% ammonia solution withabout 5% oleic acid and 5% acetone added, thereby leaving plentyof excess ammonia molecules to keep the solution alkaline.
The latest ammoniated cleaning solutions take advantage ofdetergent technologies. The oleic acid is replaced with detergentsbecause the detergents are not severely affected by hard waterswith calcium and magnesium ions.
I include the following quote verbatim from information Matthew Headrick provided me because his choice of words is excellent: "Ammonia, like alcohol and acetone, is also valuable incleaningsolutions because it is highly volatile, so the residual cleaningsolutionevaporates much faster off the cleaned surface than water alone would,preventing streaking, further oxidation, and other bad things." Therefore, there should be no fear about any ammonia residue being left behind in cast brass, which is porous, ammonia which you might fear would react with the metals after cleaning.
The reason why household ammonia effectively replaced myammoniated clock cleaning solution that I recycled (see previousessay) is because the reactions are essentially the same. The oleicacid is replaced by several different kinds of detergents (for a widevariety of grease-cutting applications), referred to on the label asanionic and nonionic surfactants. The oxide layer is similarlyremoved.
If you wanted to polish the brass plates despite the removal ofmetal atoms you could buy household ammonia from yoursupermarket. It contains about 5% aqueous ammonia, a weakalkaline, and very effective surfactants. Do not buy any cleaners that do not list the ingredients on the container. You could add a smallamount of acetone (about 5% by volume) from the same businesswhere you buy paints for your house. But please: if you do notunderstand the chemistry outlined here, buy the cleaning solutionsprovided by your clock parts suppliers. If you do not know whatyou are doing, you could easily ruin an antique clock!
My recommendation is to clean the clock parts by hand with anorganic solvent, such as tetrachloroethylene, but be careful not toinhale the fumes from the solvent, which will make you ill. Do notremove the protective oxide layer from the brass, but do removethe oxide layer off steel parts. If you prefer not to use organicsolvents because of the fumes, use the latest cleaners anddegreasers with detergents without ammonia and without acids, butthese water-based cleaners require that the clock parts bethoroughly rinsed in water and dried immediately to prevent theformation of new oxides. I dry my clock parts with an aircompressor at about sixty pounds per square inch (in the tank) verythoroughly and then in a metal box with four light bulbs (60w.) thatwarm the clock parts for a couple of hours to evaporate anyremaining traces of water.
Electrolysis:
From a theoretical point of view, it would be possible to clean the oxide layer without removing metals atoms from the surfaces if the clock parts had their oxides reduced by electrolysis. If the part were attached to the cathode and immersed into dilute sodium hydroxide solution and a potential difference of 4.5 volts (which should be enough) of direct current were applied for a couple of minutes, the metal oxides would be reduced to the metals and the oxygen ions would float away into the solution. Prolonged electrolysis would result in the gradual disappearance of the carbon anode. In my opinion, electrolysis is a lot of effort to go to only to save a few hundred metal atoms that protected the metal atoms underneath (which are then exposed to oxidation themselves). However, it is interesting to know that the removal of oxides from brass and steel parts can be achieved without the loss of metals: this should make you think of the Hall-Heroult Process, by which aluminium metal is extracted from its ore.
ACKNOWLEDGEMENTS:
"Structural and Comparative Inorganic Chemistry" by P.R.S Murray and P.R.Dawson.
2nd reprint, 1980. This was one of my A level Chemistry textbooks from Sedbergh School.
"Chemistry" by Dr. Steven Zumdahl, University of Illinois, third edition, 1993.
"Chemistry made Simple" by Dr. Fred Hess, revised edition, 1984.
Allan Headrick, B.S. (Swarthmore, Physics). Teaches chemistry
and physics and Austin High School (Austin, Texas). (Allan is my
brother). Allan provided most of the information that formed the
basis of this essay.
Dr. Steve Herron, B.S. (Heidelberg College, Ohio), Ph.D. (Iowa State University, Organic Chemistry), of Houston, Texas.
Steve is the son of Sue Wysong, a local watchmaker. Steve
answered my questions in a one-hour telephone conversation. Steve also provided much-needed help with information about complex ions of copper and zinc.
Matthew Headrick, B.S. (Princeton), graduate student at Harvard University, working on his Ph.D. in Physics. Matthew gave me some important information about solvents and about the action of soaps on lipids. (Matthew is my cousin.)
Go to Russian Clock
Clock Repair Main Page
Escapements in Motion
Links Page