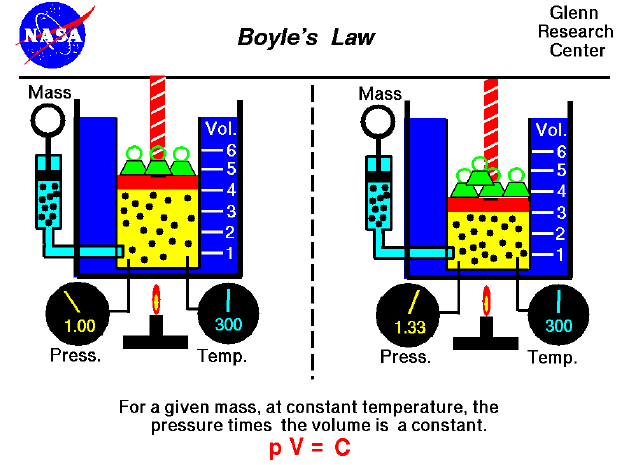
| Boyle's Law gives the relationship between volume and pressure if amount and temperature are held at constant. If the volume of a container is increased the pressure goes down, and if you put less volume the pressure goes up. What this means is that if you get a box or a container which one will have the most pressure. The container will have less pressure, and the box will have more. The box will have more because of the volume. The mathematical form of this law is; Pv=k. This means that the pressure and volume product will always be the same value if the temperature and amount remain constant. This was the relationship that Boyle discovered. |