printable table

        | 8 in third shell |
        | 8 in second shell |
  | 2 in first shell |
|
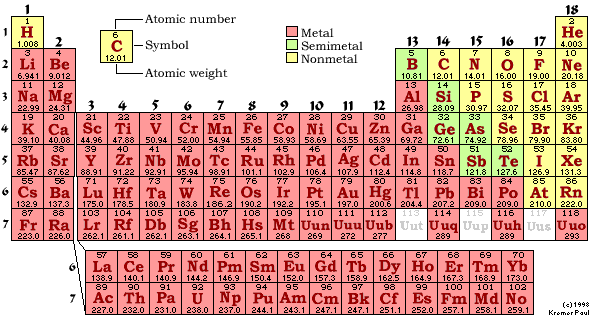
Elements that have a whole row filled are happy. They're so happy they don't need anyone else. They don't want to give electrons away or get any new ones. Because they are loners and too full of themselves to hang out with the other elements, they are kinda like royalty, or nobility. These are known as the noble gasses. Helium is one noble gas you may know. It is non-flammable and stays in gas form. You could put it with acids or run electricity through it and it wouldn't blow up or change or anything. This is because noble gasses are happy just the way they are. Neon is another noble gas. You could run electricity through it and it would make a pretty glow, but nothing else. If you look at the atomic numbers of the noble gasses and look at those rows of stadium seats, you'll notice a pattern. Row one has two seats, and helium is number two. If you add the first row plus the second row, you have the number ten, the same as neon. Add another 8 and you have eighteen, the number of argon. The noble gasses are noble because their electron rows are filled. |  |
 While some families are generous and giving, other families are takers. This family is the column next to the noble gasses, and the closer something is to being noble, the more desperately it tries to become noble. These elements almost have their orbital filled, but are one electron short. This means they'll take that electron from anything they can find.
While some families are generous and giving, other families are takers. This family is the column next to the noble gasses, and the closer something is to being noble, the more desperately it tries to become noble. These elements almost have their orbital filled, but are one electron short. This means they'll take that electron from anything they can find.