Spherical Molecules:
Diamond's Bonds
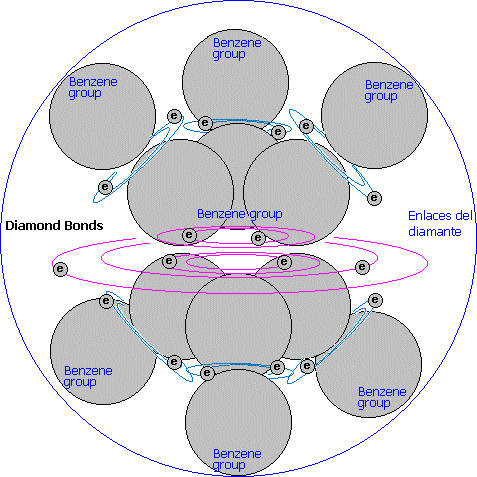
The first question to consider in diamond is its hardness and crystalline structure are due to each diamond forms a single molecule of carbon.
When staying united all their atoms for covalent bonds in their two directions (Cis-Trans) they are constituted in a single three-dimensional molecule that gives diamond its hardness and its crystalline state.
Diamond consists of hexa groups, which are constituted by a double covalent bond (benzene type): A common bond for all the atoms and a particular bond for each triad.
Apart from this, the hexa group are united among them in the following way: Each atom of the hexa groups is united by its external side with a simple connection to another hexa group forming a carbonic net in which all its connection positions are occupied.
Summarizing, each atom of carbon give two electrons for covalent bonds: one for the benzene nucleus and another for the union with another different group. In the change, this atom receives six covalent orbits for its three bonds (each covalent bonds uses two common orbits).