MNR Spectrum
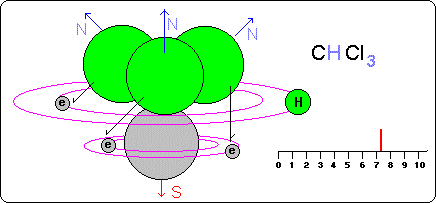
Another important factor to keep in mind is the orbits ( free to be occupied by hydrogen ) that each atom has:
--If this atom has many orbits to occupy, it means that the last orbits will be very far from the atomic nucleus and it will exercise very little influence on the hydrogen atoms. Example, carbon that has four.
--If this atom has few orbits to occupy, then the orbits (or orbit) will be very close and the atomic nucleus will exercise a lot of influence on the hydrogen atoms. Example the fluorine and chlorine that alone have one.
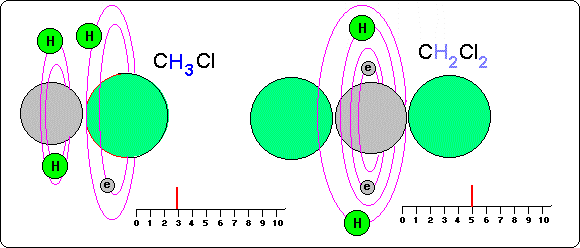
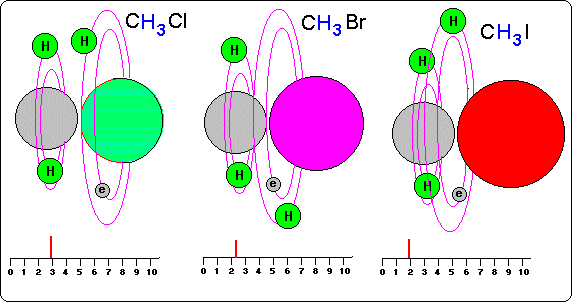
---With object of studying influence of the electronegativity in the situation of the atoms of hydrogen in the covalent connections, in this drawing we observe three compounds, methyl with halogens (CH3 Cl, CH3 Br, CH3 I).
We remember that the direct influence of electronegativity is of locating to the hydrogen atoms into the more electronegative places of the molecule, but not of powering its turn frequency.
Nevertheless, indirectly they can influence since they place them in very near positions to the nuclei of the electronegative atoms and these nuclei influence in the spin of the atoms of hydrogen.
In the drawing we see as in molecules with carbons the more electronegative atoms (p.e. Cl) situate to hydrogen more near to them than of the carbon atoms, increasing their spin potential.
On the other hand in the less electronegative atoms, hydrogen are more far from them and more near the carbons and therefore hydrogen atoms are lees influenced and with less spin.