Spherical Molecules:
Fullerene's Bonds
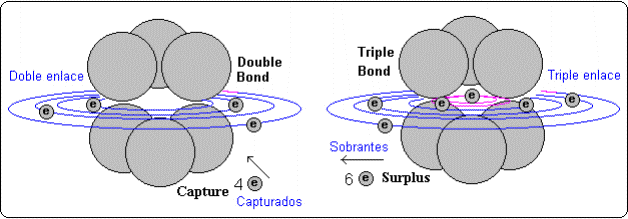
In the big molecules of pure carbon (without hydrogen) to build these molecules it is necessary to use electrons from these carbon atoms to compose the molecular bonds. This usually is got forming hexagonal structures (benzene type) and using two or more connection types, so that, while few hexa groups give other electrons, these other hexa capture the given electron to form their bonds.
This way, in the case of fullerene C60 seems that four groups hexa exist (central) with triple bonds that give six electrons each one. The other type composes six hexa groups of double bond that capture four electrons each ones. So, triples bonds give 24 electron, which are captured for the dobles bonds.
The molecular cohesion would be covalent among the carbon atoms of each hexa group and of ionic type among hexa groups, but very weak and delicate because all carbons maintain its own magnetic structure (and magnetic potential) and therefore their resultant signal Nmr should be equal in all them.
On the other hand the double bonds will have excess of electrons and they will be prone to give them what would propitiate their connections with halogens, while the triples bonds will contain less electrons and they will be favourable for the connections with alkaline.
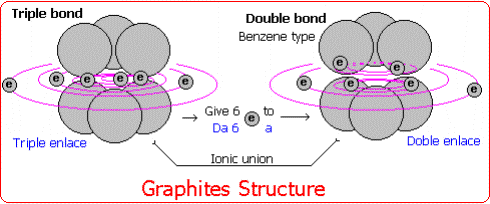
Graphite is made up of pure carbon that has a double type of structure: One of covalent character by means of which groups of six carbons (benzene type) are formed. And another of ionic character by means of which these hexa groups unite for ionic action.
For it, some hexa groups have to give electrons to other groups to be able to form the corresponding bonds.
It is gotten when forming some hexa groups with double bond and the other ones with triple bonds.
The hexas with triple bond (T) have six surplus electrons to give to the double bonds (D) that need of those six electrons.
When giving and accepting electrons these groups, they take ion character and they join alternating to form graphite (T-D-T-D-T-D.........).
I repeat again that any hexa groups have its magnetic potential almost balanced, and therefore, the cohesion among hexa groups is very weak.
Then, according to its environmental conditions of formation (pressure, temperature, etc.) graphite takes different structures, such as: spheres, spirals, tubular, lineal, etc. To more compression-more compact; while less compression-less compact.