MNR Spectrum Ethane Benzene
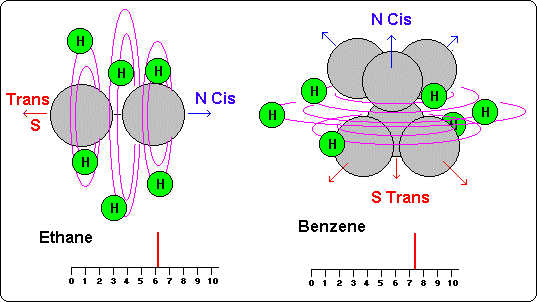
They are compared here due to their similarity in both cases in the quasi-spherical situation of the hydrogen atoms that compose them, the same number of hydrogen atoms and also for their similarity in the resultant NMR signal.
One of the accepted postulates on the NMR signals is when alone a single signal exists for all the hydrogen atoms it is because all they are topographically equivalent. I think this statement is not complete.
That is:
--If all the hydrogen atoms of a molecule were topographically equals they should have a single signal, but with the same frequency (1,2 pm about) and whose value of width (intensity) should be multiple of the existent atoms in the molecule.
And this doesn't happen in ethane neither in benzene (6 and 7 pm).
In both cases a single signal exists but with a frequency multiple of the existent atoms.
This therefore should mean that atoms form in this case a quasi-spherical group, situated appropriately to vibrate in group (like an electric bobbin), taking so, a much bigger frequency than the one that would take of making it each atom for its side.
This point of view is also supported when two atoms of hydrogen that form a triad, or even a quartet, emit us a composed signal. This is because each atom gives us a signal for itself, but the same also gives us other signal adding its frequency with the one of another next atom.
Wrong questions:
To my to understand, a questions not well developed at the present time it is when we obtain a single signal of a chemical element and we give as only solution the topographical equivalence of the different atoms to measure.
I believe this is not this way usually.
A single signal could be due to two reasons:
--1 - All atoms to measure are equivalent topographically.
In this case a single wave pick should result, but with the same frequency of a single atom and whose variation would be in the intensity of the oscillation not in its frequency.
--2.- The most common. All atoms to measure form a homogeneous group that vibrates jointly.
In this case we would obtain a single wave pick but whose frequency should be multiple number of the atoms to measure. (Ethane 6, ethene 4,4, ethyne 2,5, benzene 7, etc. )