MNR Spectrum
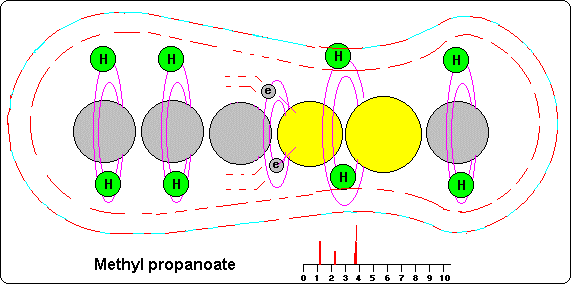
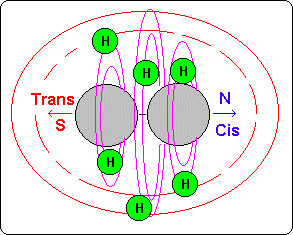
The covalence orbit (usually two) act for all and each ones of the atoms of the molecule and they consist on the union among the orbits 9 and 10 of each atom.
These orbits, which are common for all atoms, represent the layer or external �bark� of the molecule and their proximity toward the central axis (N-S) of the molecule depends on the type of atoms that in each place of that axis exists.
If we see the example of the drawing, we observe that in the atoms of carbon the covalence orbits are more distant than in oxygen because this has bigger gravitational power and their orbits are more compressed than in carbon. Therefore its orbits 9 and 10 are more near to the nucleus than in the atoms of carbon.
This circumstance makes that the atoms of hydrogen that circulate for these layers are attracted by the electronegative oxygen, so they are much nearer to the atomic nuclei than in the molecules where alone the carbon exists.
For this reason the hydrogen atoms near to oxygen (or other) they are inside bigger gravitational and magnetic fields that produce them a bigger magnetic power and therefore they develop a signal of more frequency.