MNR Spectrum
To begin we can give a simple definition of both terms:
�In the covalent molecules electro negativity is that situates and distributes to the hydrogen atoms, and the magnetic potential is the intensity of the magnetic fields of the place where they are situated and that gives hydrogen its magnetic potential and its level of frequency nmr.�
The spin or turn of the hydrogen atoms is accelerated by the magnetic fields of other nearer atoms.
To bigger magnitude of an atom-bigger will be its magnetic potential and its spin frequency.
So, in hydrogen proximity to the big atoms produces bigger spin or turn frequency than proximity to small ones.
Therefore it is necessary to keep in mind both factors to be able to study the nmr signal.
--If an atom is very electronegative, this will attract very near the atoms of hydrogen.
--If an atom is of great magnitude, this will produce a high spin value in hydrogen.
Therefore we have to observe situation of the hydrogen atoms and magnetic potential in this position due to the magnitude of the nearest molecular atoms.
Beside, electronegativity only influence on the hydrogen atoms because alone they are attracted on the electronegative ones to be hydrogen orbital behaviour.
On another atoms only the magnetic potential acts. So that, for other atoms
we have to be in mind proximity, magnitude, grouping shape and cluster number of these atomos, but not electronegativity.
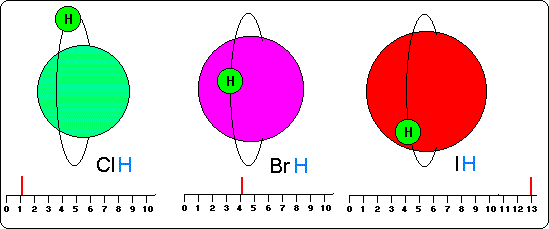
In the drawing of this page we see firstly influence that the magnitude of the atoms of a molecule produce on the hydrogen atoms that occupy their saturation orbits.
It is shown in this drawing the halogen acids of a single component and a single atom of hydrogen for their saturation (ClH, BrH and IH).
We observe that to bigger magnitude of an atom-bigger magnetic influence on the hydrogen and bigger turn frequency or spinning. But also, and because the orbits of big atoms are more compressed, the hydrogen atoms are more near to the nucleus in the big atoms than in the smallest ones.
Therefore and summarizing, to bigger magnitude of the atoms of the molecule--bigger magnetic potential transmitted to the atoms of hydrogen, taking place in them a bigger turn frequency or spin ( nmr signal ).