|
AGOSTO 2.003
AUGUST 2.003
VALACYCLOVIR, ISOTRETINOIN, NIMESULIDE, ORLISTAT, DUTASTERIDE,
HEREDITARY
POIKILODERMATOUS SYNDROMES

1.)
FDA Approves Valtrex for Reducing Risk For Sexual Transmission With
Suppressive Therapy.
2.)
Investigan droga contra el acné (Roaccutan) en casos de depresión y suicidios.
3.) Mother of Suicidal Tampa Pilot
Says She Will Sue Maker of Accutane
4.) Mother of 15-year-old Tampa suicide pilot
blaming acne drug for boy's death.(Roaccutane)/ 147 intentos de suicidio reporta FDA !!!
5.)
One year later, teen's mother, friend haunted by plane crash death./ Roche Demandado por 70 millones de dolares por madre del joven que se suicido al estrellar avioneta
6.) Constipation, polyuria, polydipsia, and edema associated with
orlistat/ (XENICAL.) efectos adversos.
7.)
Orlistat e hipertensión /
Xenical e hipertension.
8.) INDIA: MEDICAMENTO VINCULADO A MUERTES DISPONIBLE EN EL
MERCADO (NIMESULIDE= AULIN, AINEX, SCAFLAN)
9.)
WHAT IS DUTASTERIDE (AVODART™ AVOLVE™) /Nueva medicina para la caida del cabello
10.)
AVODART™ / DUTASTERIDE/ The Biggest News In Hair Loss Treatment Since Propecia
11.)
Synopsis of Hereditary Poikilodermatous syndromes

1.) FDA Approves Valtrex for Reducing Risk For Sexual
Transmission With Suppressive Therapy.
FDA Updates Labeling of Valtrex source: Aug 29, 2003 FDA
Talk Paper
Source: http://www.natap.org/
The Food and Drug Administration (FDA) has approved a new indication for
Valtrex (valacyclovir hydrochloride) Caplets; Valtrex reduces the risk of
heterosexual transmission of genital herpes to susceptible partners with healthy
immune systems when used as suppressive therapy in combination with safer sex
practices.
Genital herpes is a common sexually transmitted infection caused by herpes
simplex viruses (HSV). Many individuals have no or only minimal signs or
symptoms from genital HSV infection and may transmit the virus during sexual
contact when they show no signs of active infection (i.e. genital lesions).
The following safer sex practices can also lower the chances of passing genital
herpes to a partner:
--Use a condom made of latex or polyurethane when you have sexual contact. --Do
not have sexual contact with your partner when you have any symptom or outbreak
of genital herpes.
FDA based its decision to revise the labeling for Valtrex on the results of an
international, double-blind, placebo-controlled clinical trial conducted by the
manufacturer, GlaxoSmithKline (GSK) of Research Triangle Park, N.C. The study
was conducted among about 1500 monogamous, heterosexual couples and lasted eight
months. At the beginning of the study, only one member in each couple had
evidence of genital herpes. The results of the GSK study showed a 48% reduction
in HSV acquisition; individual results may vary based on consistency of safer
sex practices.
Valtrex may cause kidney problems in some people. In addition, Valtrex may cause
nervous system problems; these include aggressive behavior, unsteady movements,
shaky movements, confusion, speech problems, hallucinations, seizures and coma.
Kidney and nervous system problems have happened in patients who already have
kidney disease and in elderly patients whose kidneys do not work well due to age.
Therefore, it is important for patients to tell their healthcare providers if
they have kidney problems or other medical conditions before taking Valtrex.
Today’s action follows the recommendation of FDA’s Antiviral Drugs Advisory
Committee, which met on May 14, 2003, to discuss GSK’s then-proposed use of
Valtrex for reduction of the risk of transmission of genital herpes with the use
of suppressive therapy. FDA first approved Valtrex in 1995.
To read report on FDA Hearing and review of study results:
Valtrex For Reducing Transmission of Genital Herpes- FDA Hearing: FDA panel
votes 11-0 to recommend approval http://www.natap.org/2003/may/051503_1.htm
CLINICAL TRIALS Herpes Zoster: Two randomized double-blind clinical trials in
immunocompetent adults with localized herpes zoster were conducted. VALTREX was
compared to placebo in patients less than 50 years of age, and to ZOVIRAX in
patients greater than 50 years of age. All patients were treated within 72 hours
of appearance of zoster rash. In patients less than 50 years of age, the median
time to cessation of new lesion formation was 2 days for those treated with
VALTREX compared to 3 days for those treated with placebo. In patients greater
than 50 years of age, the median time to cessation of new lesions was 3 days in
patients treated with either VALTREX or ZOVIRAX. In patients less than 50 years
of age, no difference was found with respect to the duration of pain after
healing (post-herpetic neuralgia) between the recipients of VALTREX and placebo.
In patients greater than 50 years of age, among the 83% who reported pain after
healing (post-herpetic neuralgia), the median duration of pain after healing
[95% confidence interval] in days was: 40 [31, 51, 43 [36, 55], and 59 [41, 77]
for 7-day VALTREX, 14-day VALTREX, and 7-day ZOVIRAX, respectively.
Genital Herpes Infections: Initial Episode: Six hundred and forty-three
immunocompetent adults with first episode genital herpes who presented within 72
hours of symptom onset were randomized in a double-blind trial to receive 10
days of VALTREX 1 gram twice daily (n = 323) or ZOVIRAX 200 mg 5 times a day (n
= 320). For both treatment groups: the median time to lesion healing was 9 days,
the median time to cessation of pain was 5 days, the median time to cessation of
viral shedding was 3 days.
Recurrent Episodes: Three double-blind trials (2 of them placebo-controlled) in
immunocompetent adults with recurrent genital herpes were conducted. Patients
self-initiated therapy within 24 hours of the first sign or symptom of a
recurrent genital herpes episode. In 1 study, patients were randomized to
receive 5 days of treatment with either VALTREX 500 mg twice daily (n = 360) or
placebo (n = 259). The median time to lesion healing was 4 days in the group
receiving VALTREX 500 mg versus 6 days in the placebo group, and the median time
to cessation of viral shedding in patients with at least 1 positive culture (42%
of the overall study population) was 2 days in the group receiving VALTREX 500
mg versus 4 days in the placebo group. The median time to cessation of pain was
3 days in the group receiving VALTREX 500 mg versus 4 days in the placebo group.
Results supporting efficacy were replicated in a second trial.
In a third study, patients were randomized to receive VALTREX 500 mg twice daily
for 5 days (n = 398) or VALTREX 500 mg twice daily for 3 days (and matching
placebo twice daily for 2 additional days) (n = 402). The median time to lesion
healing was about 4.5 days in both treatment groups. The median time to
cessation of pain was about 3 days in both treatment groups.
2.) Investigan droga contra el acné (Roaccutan) en casos de depresión y suicidios
El medicamento se comercializa en Chile bajo receta médica retenida
Source:
http://www.geocities.com/econatsalud/noticias/
Pedro Vicario
Caso del joven norteamericano que estrelló su avioneta
contra un edificio en EE.UU., quien se presume consumía el medicamento, revivió
dudas contra el fármaco.
Charles Bishop, el adolescente de 15 años que el
pasado sábado -emulando los atentados al World Trade Center- estrelló un avión
Cessna contra el edificio del Bank of America Plaza en Tampa, Florida, era un
joven normal. Tan normal que tenía los problemas propios de los jóvenes de su
edad. En el hogar del novel piloto fue encontrada una receta prescrita a su
nombre con el fármaco Accutane, versión norteamericana del medicamento vendido
en Chile Roacnetan, que se usa comúnmente para el tratamiento del acné juvenil.
El hecho volvió a sacar al tapete el tema de los riesgos que esta droga pudiera
tener en la población. A las ya conocidas contraindicaciones a embarazadas por
riesgo de malformaciones y muertes de sus hijos (advertidas debidamente en su
envase), se agrega la sospecha de que el fármaco pudiera ocasionar depresión e
incluso intentos de suicidio. Ese podría ser el caso del joven kamikaze
norteamericano quien, a juzgar por la receta encontrada en su casa, era un
típico consumidor de la droga.
El Accutane -su nombre genérico es isotretinoína- se vende en Chile con receta
médica retenida bajo el nombre de Roacnetan. El laboratorio Roche, productor del
fármaco, aclaró a través de la vicepresidenta del laboratorio farmacéutico
Hoffman-La Roche, Carolyn Glynn, que la empresa estaba tomando el caso “muy
seriamente”. “Ciertamente que vamos a analizar este caso detenidamente” agregó
Glynn, ante la magnitud que pudiera tomar el problema, tomando en cuenta que el
medicamento es recetado a pacientes de todo el mundo.
¿Qué pasa en Chile?
Según el psiquiatra José Bitrán, el ha tenido dudas respecto de pacientes
puntuales que han tomado el Roacnetan. “El paciente de uno que tiene problemas
anímicos y a su vez es un adolescente, en la mitad del tratamiento empieza a
tomar el fármaco, comienzan ha empeorar los síntomas y se genera un
entorpecimiento de la evolución del cuadro” opinó.
Concordó en que si bien no se puede afirmar que el Roacnetan sea la causa del
problema, ya que no existen estudios científicos al respecto, si se logra
establecer una relación entre casos de depresión y uso del fármaco.
Héctor Fuenzalida, dermatólogo del Hospital Clínico de la Universidad de Chile,
recomendó antes de recetarlo “tener la precaución, en pacientes con depresión o
esas características, de recetar el Roacnetan. Yo dudaría en hacerlo o quizás
controlaría la dosis del fármaco mucho más seguido, con un apoyo psiquiátrico
detrás”.
El médico, que si bien prefirió relacionar la depresión a la baja autoestima que
genera el acné, dijo que ante la duda es mejor tomar en cuenta las críticas que
existen sobre el medicamento. Esto pese a que el fármaco “es un medicamento
aprobado por la FDA y cumple todas las normas” concluyó el especialista.
3.) Mother of Suicidal Tampa Pilot Says She Will Sue Maker of
Accutane / Madre del joven que se suicido intentara accion legal contra el
fabricante de Accutane (Roche).
Source:
http://www.injuryboard.com/
April 16, 2002
The mother of Charles Bishop, the 15-year-old pilot who committed suicide on
January 5 by flying a stolen Cessna into a Tampa high rise, says Accutane is
responsible for her son's death. Julie Bishop recently told reporters in an
interview that because her son had shown no signs of depression before the crash,
"the only conclusion we have been able to draw is the Accutane poisoned him."
Bishop said she plans to file suit against the drug's maker.
A prescription for Accutane, made by Hoffman-La Roche, was found in Bishop's
home during a police search shortly after the crash. Although an autopsy did not
find trace amounts of Accutane in Bishop's blood, critics claim that the
controversial medication can have "depressive effects on the brain" weeks after
a person stops using it.
4.) Mother of 15-year-old Tampa suicide pilot
blaming acne drug for boy's death / 147 pacientes reporta FDA con intentos de
suicidio por Roaccutane desde su debut en el mercado.
Source: http://www.injuryboard.com/
April 17, 2002, Wednesday
Associated Press
By VICKIE CHACHERE
The maker of the acne drug Accutane faces a $70 million lawsuit claiming the
medicine caused severe psychosis in a 15-year-old boy who crashed a stolen plane
into a Tampa high-rise. The lawsuit, filed against Hoffmann-La Roche on Monday
by the family of Charles Bishop, blames Accutane for the boy's suicide. Bishop
slammed the small plane into the high rise on Jan. 5 and left behind a note
expressing sympathy for Osama bin Laden and supporting the Sept. 11 attacks.
In an interview aired Tuesday by NBC's ``Today'' show, the boy's mother, Julia
Bishop, said her son had showed no signs of depression.
``This child was a happy, well-balanced, forward-thinking child who had a great
deal to live for,'' Bishop said. ``This was psychotic and the only conclusion we
have been able to draw is the Accutane poisoned him.''
A Hoffmann-La Roche spokeswoman said the company was unaware of the lawsuit and
does not believe the drug is dangerous.
Accutane carries a warning about suicide, but the company points to statistics
showing sufferers of severe acne and teen-agers the main users of the drug
generally have higher suicide rates.
``We continue, as do the experts, to believe there is no link,'' said company
spokeswoman Carolyn Glynn.
The Food and Drug Administration says 147 people taking Accutane either
committed suicide or were hospitalized for suicide attempts from 1982 to May
2000. An estimated 13 million patients have used Accutane since its debut in
1982.
An autopsy found no trace of Accutane in Bishop's blood, but attorneys for the
family say so much blood was lost in the crash that the test may not have been
useful.
Department of Diagnostic Sciences and Pathology, Dental School, University of
Maryland, Baltimore 21201-1586, USA. [email protected]
BACKGROUND: The authors provide clinical findings in five patients wearing oral
jewelry to illustrate the risks of experiencing periodontal injury associated
with body piercing involving intraoral and perioral sites. They also present a
literature review of other adverse dental and medical consequences attributed to
oral piercing. CASE DESCRIPTIONS: Five young adult patients with tongue and lip
piercing sought dental care. Each patient exhibited some degree of gingival
recession and mucogingival defects in proximity of their oral jewelry. Three of
these patients had probing depths ranging from 5 to 8 millimeters in the
affected areas. CLINICAL IMPLICATIONS: Intraoral and perioral jewelry may be
associated with the development of significant mucogingival deformities. Because
severe attachment loss can develop even when gingival recession is minimal, it
is critical that patients with oral piercing routinely undergo comprehensive
periodontal assessment. The authors urge clinicians to educate patients about
the potential risks regarding the practice of oral piercing.
5.)
One year later, teen's mother, friend haunted by plane crash death/Madre de
joven que murio al estrellar avioneta demanda al laboratorio Roche por 70
millones de dolares !!!
Source: http://www.injuryboard.com/
Associated Press
January 06, 2003, Monday
One year after 15-year-old Charles Bishop crashed a stolen airplane into a Tampa
skyscraper, his mother and best friend said they are still haunted by death and
struggling with the public attention it has drawn. Julia Bishop, who has filed a
$70 million lawsuit against the maker of the acne medication Accutane that her
son was taking before the crash, has made few public statements since her son's
death Jan. 5, 2002.
``This is a news story for the rest of the world,'' she told the St. Petersburg
Times in a story for Sunday's edition. ``But for me, he was my life and my son
and it's extremely difficult to have this thrown into my face all the time.''
Bishop is limited in what she can talk about because of the lawsuit, which
contends Accutane caused the boy to develop severe psychosis and led him to
commit suicide. A trial is set for March 2004 in U.S. District Court in Tampa.
Julia Bishop remembers her son, a high school freshman who dreamed of becoming
an Air Force Pilot, excitedly talking about an upcoming trip to Australia and
New Zealand as a student ambassador.
Days later, he left a two-page note, expressing sympathy for Osama bin Laden and
supporting the Sept. 11, 2001, terrorist attacks. He also claimed he had
resisted recruiting attempts by al-Qaida terrorists.
``When you see a pattern and then suddenly for somebody to snap and think they
are working for Osama bin Laden ... I'm sure you've seen the note,'' Julia
Bishop said. ``That's really sick.''
Julia Bishop said she meets about once a month with her son's best friend,
Emerson Favreau, who says he still feels guilty he didn't notice any suicide
warning signs in his friend.
Two days before the crash, Charles sent Favreau an e-mail saying he was going to
be on the news. Charles ended the online conversation by saying: ``See you
Monday.''
``That's what made me mad,'' Favreau said. ``Because he knew he wasn't going to
see me on Monday.''
Favreau has re-created his friend's final flight along Tampa's skyline on an
airplane computer simulator.
``I was trying to figure out maybe if there was something going on,'' Favreau
said. ``I still couldn't believe that he did it on purpose.''
Emerson said he was finally convinced by a federal report in November that said
Bishop flew dangerously close to the MacDill Air Force Base control tower before
crashing into the 42-story Bank of America Plaza in downtown Tampa.
``Before, there was nothing concrete that he had done it on purpose,'' Favreau
said. ``But you can't accidentally (buzz a control tower) with a Cessna.''
Now a junior at East Lake High School, Favreau said students don't talk much
about Charles Bishop anymore, but that he is still haunted by the suicide.
``It's just something that you can't get off your head,'' Favreau said.
6.) Constipation, polyuria, polydipsia, and edema associated with
orlistat/ (XENICAL.) efectos adversos.
Ann Pharmacother. 2002 Jul-Aug;36(7-8):1168-70.
Packard KA, Wurdeman RL, Reyes AP.
Creighton Cardiac Center, Omaha, NE 68131-2044, USA. [email protected]
OBJECTIVE: To report the occurrence of a novel group of adverse effects
associated with initiation and rechallenge of orlistat. CASE SUMMARY: A 42-year-old
white woman developed symptoms of constipation, polyuria, polydipsia, and
increased lower-leg edema after 2 weeks of treatment with orlistat 120 mg 3
times daily. The drug was discontinued for 4 days and the symptoms resolved. On
reinstitution of the orlistat treatment, the symptoms reappeared within 2 days.
Thereafter, the medication was permanently discontinued. DISCUSSION: Common
gastrointestinal adverse reactions associated with orlistat use include fecal
urgency and abdominal pain and discomfort. Pedal edema has also been reported to
occur, although less frequently. No reports were discovered documenting the
occurrence of constipation, polydipsia, and polyuria associated with the use of
orlistat. Despite careful consideration of other possible causes of these
symptoms, the temporal association between initiation, discontinuation, and
rechallenge of orlistat and the patient's symptoms suggest a medication-related
adverse event. Based on the Naranjo probability scale, the likelihood that
orlistat was the cause of this cluster of adverse effects is possible.
CONCLUSIONS: It is important for the healthcare provider to be aware of these
adverse effects to promptly evaluate and differentiate between possible causes
of similar reactions.
7.) Orlistat e hipertensión /
Xenical e hipertension.
Source: http://www.fonendo.com/
British Medical Journal 10/07/2000
El centro de Farmacovigilancia suizo ha reportado un caso de una paciente que
hubo de retirar el fármaco por HTA.
Sin antecedentes de interés, comenzó el fármaco hasta llegar a una dosis de 120
mg/8 h. Comenzó a presentar mareo, edemas periféricos y cefalea pulsátil,
constatándose unas cifras de TA de 190/100 mm Hg en tres ocasiones.
Tras retirar el fármaco, volvió a sus cifras normales. Tras reintroducirlo,
volvió a presentar la misma sintomatología, por lo que se retiró
definitivamente. La paciente se volvió a recuperar.
El fabricante había comunicado 13 casos previos de HTA, pero la información era
incompleta. Aún no disponemos de recomendaciones al respecto, pero quizás sea
necesario controlas las cifras de TA en los pacientes en tratamiento.
8.) INDIA: MEDICAMENTO VINCULADO A MUERTES DISPONIBLE EN EL
MERCADO (NIMESULIDE= AULIN, AINEX, SCAFLAN)
Source:
http://www.boletinfarmacos.org/
Sanjay Kumar, BMJ, 2003; 326:70
El nimesulide, un anti-inflamatorio no esteroideo, se ha retirado de muchos
mercados europeos pero sigue estando disponible en India. Se sabe que es un
medicamento hepatotóxico y que ha provocado la muerte a varios niños. La India
aprobó este medicamento en 1994 para uso en casos de inflamación
musculoesquelética, sin embargo se utiliza frecuentemente como analgésico y
antipirético.
Según el Dr Chandra Mohan Gulhati, editor del Boletín de Información sobre el
Medicamento de India, el medicamento no está aprobado en EE.UU., Canadá,
Australia, y partes de Europa; y Finlandia, España y Turquía lo retiraron del
mercado el año pasado.
Después de que los medios de comunicación denunciaran el hecho, el responsable
de controlar los medicamentos en India, Ashwini Kumar, dijo que el gobierno
designaría a un comité de expertos para que estudiase el problema. El asistente
del Sr. Kumar, Ram Teke, dijo a BMJ que el medicamento sigue estando disponible
y que no había intención de reconsiderar su utilización o de retirarlo del
mercado. El volumen de ventas de este medicamento es del orden de 1900 millones
de rupíes (39,5 millones de dólares).
El nimeluside además de estar disponible como producto activo único, también
esta presente en otros 30 medicamentos y en gotas para niños menores de un año.
Todas estas combinaciones son ilegales porque no cuentan con el registro
correspondiente.
El Dr. Gulhati dice que no hay un buen sistema para informar sobre reacciones
adversas en India. Hay 12 medicamentos que se han retirado de los mercados
globales, o que son de prescripción limitada, que están disponibles en la India,
estos son: anagen, cerivastatina, droperidol, furazolidone, lynestrenol,
nitrofurazona, fenformin, fenolftaleina, fenilbutazona, piperazina, y
quiniodoclor.
Es más, cuando se decide retirar un medicamento del mercado, la implementación
de esta decisión es muy deficiente, dijo el Dr Gulhati. Por ejemplo, en junio
pasado se tomo la decisión de retirar los antialérgicos astemizole y terfenadine;
la notificación no se hizo hasta octubre y en ella se indicaba que no se
retirarían hasta agosto del 2003.
Según el Dr. Gulhati los medicamentos deberían retirarse del mercado tan pronto
como se determina que son nocivos, y la única explicación plausible es que hay
interés en proteger a la industria. Dice que las decisiones de la autoridad
reguladora están afectadas por los intereses comerciales de los cabilderos de la
industria y la corrupción; y que es una autoridad a la que no se le exige
transparencia ni que justifique sus actividades.
Las acciones de los medios de comunicación han provocado que dos laboratorios
recorten sus volúmenes de producción de nimesulide.
Ajay Kumar Handa, presidente de marketing para Centaur Pharmaceutical, dijo: "
Ya no estamos produciendo jarabe de nimesulide." Y añadió “Por lo que yo sé no
hay problemas con la administración de este medicamento a adultos.”
El grupo Social Jurist presentó un caso a la Corte Suprema de Delhi donde
cuestiona la disponibilidad de nimesulide y de otros medicamentos que se han
retirado de otros mercados pero que siguen estando disponibles en India.
Krezel W, Ghyselinck N, Samad TA, et al. Impaired locomotion and dopamine
signaling in retinoid receptor mutant mice. Science. 1998;279:863-867.
9.)
WHAT IS DUTASTERIDE (AVODART™ AVOLVE™) /Nueva medicina para la caida del cabello
!!!
Source:
http://www.dutasteridedirect.com/
Dutasteride (Avodart, Avolve) - a new, emerging treatment for hair loss
DutasterideDIRECT.com is your online source for news and information on the
latest treatments for hair loss. This FAQ has been compiled from a number of
sources to give an overview of the use and effects of Dutasteride.
What is Dutasteride (Avodart, Avolve)?
Avolve is the European brand name for Dutasteride, an oral medicine made by
GlaxoSmithKline for treating symptomatic benign prostatic hyperplasia (BPH) in
men.
The active compound in Avolve, Dutasteride, has the added benefit of treating
genetic male pattern hair loss on the vertex (top of the head) and the mid-scalp
area. Dutasteride is chemically similar to Finasteride, the active compound in
Propecia.
Avolve is also known by its US brand name, Avodart. The medicine has been
approved by the US Food & Drug Administration, and in Europe, for treating BPH.
Avolve is supplied in 0.5mg soft gelatin capsules.
How does Avolve work?
Researchers have discovered that men who suffer from benign prostatic
hyperplasia (BPH), or from male pattern hair loss, have increased levels of the
hormone dihydrotestosterone (DHT).
DHT is produced from testosterone by an enzyme called 5-alpha-reductase.
Biochemical analysis reveals higher levels of 5-alpha-reductase in the
bloodstream of men with BPH, and in the scalps of men with hair loss; and less
of this enzyme in men with no BPH or no hair loss.
Avolve inhibits 5-alpha-reductase, blocking the formation of DHT. This
interrupts a key trigger element in both BPH and development of male pattern
hair loss.
What is dihydrotesterone (DHT)?
DHT is one of several male hormones in the body. DHT is responsible for the
development of the external genitals in the male foetus. However, in adult males
DHT appears to cause:
male pattern hair loss
prostate enlargement
shortening of the growing phase of hair
progressive miniaturisation of hair follicles
decreasing number of visible hairs
acne
How is Avolve different from Propecia?
Both medicines work in a similar way. However, Avolve (Dutasteride) inhibits the
activities of two types of 5-alpha-reductase enzymes. In contrast, Propecia (Finasteride)
only inhibits one type. Avolve has been shown to decreases levels of DHT by 90%
after only two weeks, making it a more powerful and faster-acting weapon against
BPH and hair loss than Finasteride.
What studies and trials have been done concerning Avolve?
A total of 2951 men with moderate to severe BPH were treated with 0.5 mg Avolve
(dutasteride) daily. The study found that acute urinary retention was reduced by
57%, and the risk of benign prostatic hyperplasia-related surgical intervention
was cut by 48% compared with placebo. The drug was shown to be well tolerated.
GlaxoSmithKline also completed Phase II trials for FDA approval of Avolve for
treating hair loss. After six month of treatment, the hair counts measured in a
1 inch diameter circle increased by an average 96 hairs with 0.5mg Avolve daily,
compared to an average 72 hairs with 5mg Propecia (Finasteride) daily.
So these initial trials show that Avolve is around 30% more effective than
Propecia in promoting hair regrowth. However, please note that Avolve has only
been specifically approved for treating BPH. It has not yet been approved
specifically for treating hair loss.
When will Avolve be approved for treating hair loss?
In November 2002 GlaxoSmithKline cancelled its planned Phase 3 trials for Avolve
for treating hair loss. The company has not publicly given a reason for this.
Industry sources speculate that the trials were stopped because the maker thinks
Avolve will be perceived as too similar to Propecia in consumers' minds, and may
not generate sufficient return on investment to justify the cost of the
approvals process as a treatment for hair loss.
However, Avolve is approved by the US FDA and by European bodies for the
treatment of BPH, and so has passed all relevant safety standards.
Will Avolve help hair re-growth for all men?
As with Propecia, Avolve increases the number of scalp hairs, helping to fill-in
thin areas of the scalp. Although results will vary, generally men will not re-grow
all of the hair they have lost. Male pattern hair loss occurs gradually over
time, but Avolve can significantly reduce or delay hair loss. Note that Avolve
is not yet approved for treating hair loss: its effects are a side-effect of its
actions against BPH.
Is Avolve safe?
Clinical trials showed that it was generally well tolerated. Most side effects
were mild or moderate and generally went away while on treatment in both the
Avolve and placebo groups.
Drug-related side effects during the first six months were as follows:
impotence (4.7% vs. 1.7% for placebo)
decreased libido (3% vs. 1.4%)
breast tenderness and breast enlargement (gynecomastia; 0.5% vs. 0.2%)
ejaculation disorders (1.4% vs. 0.5%).
Avolve should not be used in women and children. Women who are pregnant or may
become pregnant should not handle this medicine because of possibility of
absorption and subsequent potential risk to a male foetus.
Men treated with Avolve should not donate blood until at least six months after
their final dose to prevent the medicine going to a pregnant woman through a
blood transfusion.
Men with liver disease should talk to their doctor before taking Avolve.
10.) AVODART™ / DUTASTERIDE/ The Biggest News In Hair Loss
Treatment Since Propecia.
Source: http://www.hairsite2.com/
FDA clearance for Avodart or dutasteride was granted to GlaxoSmithKline on
October 9, 2002. This is the biggest news in hair loss treatment since Propecia.
This page is updated regularly based on discussions in DUTASTERIDE FORUM. If you
want the latest discussions, debates and developments about Avodart or
Dutasteride, visit the DUTASTERIDE FORUM. But if you just want to read about
forum summaries and important issues about Avodart or Dutasteride on an ongoing
basis, this page will be very helpful. Most information here are courtesy of
posters in the Dutasteride Forum.
WHAT IS AVODART OR DUTASTERIDE?
After 30 Dutasteride forums in HairSite and endless speculation for almost two
years, Dutasteride is finally a reality under the brand name Avodart™ !
The FDA approval for NDA#21-319/s-001 was given on October 9, 2002 and the label
was posted on the same day.
This is the biggest news in hair loss treatment since FDA's clearance for
Propecia in December 1997. The interesting thing is that Avodart is NOT a hair
loss drug !!
Avodart™ (Dutasteride) is approved for the treatment of Benign Prostatic
Hyperplasia (BPH). Not to be mistakened with prostate cancer, BPH is a non-cancerous
enlargement of the prostate glands. It is fairly common among older men aged 50
or above. BPH usually manifest in a variety of lower urinary tract symptoms (LUTS)
such as urinary frequency, urgency, nighttime urination, decrease in urinary
stream, incomplete emptying and incontinence etc.
Avodart is a synthetic 4-azasteroid compound. It is the first FDA approved
medication that can inhibit both types of 5-alpha reductase (5AR), an enzyme
that is commonly believed to responsible for the conversion of testosterone to 5
alpha-dihydrotestosterone (DHT). It is widely suggested that DHT is the main
androgen responsible for prostate growth as well as male pattern baldness.
ACTIVE INGREDIENTS
Avodart standard dosage is 0.5mg of the active ingredient dutasteride per soft
gelatin capsule. The capsule is compounded by dissolving 0.5mg of dutaseride in
a combination of mono-di-glycerides caprylic/capric acid and butylated
hydroxytoluene. Other ingredients include BSE-free bovine gelatin, glycerin and
yellow ferric oxide.
TOPICAL DUTASTERIDE
If you are able to obtain Dutasteride in its raw form, you might even be able to
compound your own Dutasteride topical solution. It was reported in Avodart's
prescribing information that the medication is absorbed through the skin as well.
Dutasteride in its raw form is a white-yellowish powder. It is insoluble in
water but can easily be dissolved in ethanol (44mg/mL), methanol (64 mg/mL) and
polyethylene glycol 400 (3mg/mL). However, please note that effectiveness of
Dutasteride or Avodart as a hair loss treatment has not been clinically
established.
You may want to follow this thread for more thorough discussion about topical
dutasteride.
DUTASTERIDE vs FINASTERIDE
Unlike Finasterdie (Propecia or Proscar) which only inhibits Type II 5AR,
Dutasteride functions as a dual 5AR inhibitors and have been proven to be more
effective as an treatment for BPH and possibly pattern hair loss or baldness.
According to GSK, Dutasteride has shown to exhibit significantly less inter-individual
variation in the level of DHT suppression compared to Finasteride.
A comparison between Avodart (Dutasteride) and Proscar (Finasteride 5 mg) can be
found in Dutasteride forum.
STANDARD DOSE
Scientists have evaluated the effectiveness of Dutasteride at different daily
dosage, ranging from 0.01 mg, 0.05 mg, 0.5 mg, 2.5 mg, and 5.0 mg per day. It
was found that the highest suppression of DHT was achieved with 2.5 mg or 5.0 mg
per day. At a daily dosage of 2.5 mg or 5.0 mg, Dutasteride suppresses close to
100% of the DHT whereas 5.0 mg daily dosage of Finasteride only suppresses close
to 70% of the DHT. According to information provided by GSK, the level of DHT
suppression does not seem to different significantly between 2.5 mg and 5.0 mg.
And at a daily dose of 0.5mg, DHT inhibiton is close to 90%. Please also read
below OPTIMAL DOSE FOR HAIR LOSS.
MAXIMUM EFFECT
According to Avodart or dutasteride's prescribing information, the maximum
effect of dutasteride can be observed within 1 to 2 weeks. "After 1 and 2 weeks
of daily dosing with dutasteride 0.5mg, median serum DHT concentrations were
reduced by 85% and 90% respectively." It was further reported that test patients
treated with a daily dosage of 0.5mg dutasteride for 2 years, the median
decrease in serum DHT concentration was over 92% for both year 1 and year 2 of
the 2-year trial.
SIDE EFFECTS, WARNINGS, CONCERNS
1) Women or children should not use Avodart™ or dutasteride.
2) Women who are pregnant or may be pregnant should not handle Avodart™ for fear
of potential risks to a male fetus.
3) No measurable bone mineral density level change was observed in test patients
after a 52 weeks trial.
4) Men using Avodart™ or dutasteride should not donate blood until at least 6
months after the medication was last used for fear of possible administration of
dutasteride tainted blood to pregnant female transfusion patient.
5) Men with liver problems should not use Avodart™.
6) Patients' ejaculate volume might decrease as a result of the medication. A
small number of the test patients experienced impotence and a reduction in
libido.
7) No clinically significant change in hormone levels were observed in test
patients.
8) No clinically significant changes in sperm concentration, sperm motility or
sperm morphology although some subjects reported reduction in ejaculate volume.
9) A 2-year statistical summary showed that sexual adverse events such as
impotence, decreased libido, and ejaculation disorder decreased with the
duration of the treatment. Fewer incidences of adverse sexual events were
reported over time.
OPTIMAL DOSAGE FOR HAIR LOSS
For the treatment of BPH, the official recommendation from GSK is to take 1 x
0.5mg capsule orally every day with or without food.
However, it appears that for treating hair loss, a daily dose of 2.5mg daily
seems to generate the best results. See Dutasteride Results.
Also, please follow this thread in Dutasteride Forum which discussed the effect
of Avodart on serum DHT vs scalp DHT.
0.5mg Dutasteride inhibits ~ 92% serum DHT and 55% scalp DHT
2.5mg Dutasteride inhibits ~ 95% serum DHT and 82% scalp DHT
DarkMage and Bryan in the Dutasteride forum have also provided very valuable
information about the optimal daily dose for dutasteride. You can find their
charts and analysis at "Optimal Daily Dose".
WITH OR WITHOUT FOOD
Please read Bryan's post and his interpretation about whether Avodart should be
taken with or without food.
GENUINE AVODART™
Please note that genuine Avodart™ are oblong, opaque, dull yellow, gelatin
capsules with the word "GX CE2" clearly printed in red on one side. Avodart™ is
packaged in bottles of 100 (NDC 0173-0712-00) with child resistant closures. You
may also find genuine Avodart™ in blister packs of 70 capsules (NDC
0173-0712-01).
STORAGE
15-30 degrees Celsius or 59 - 86 degrees F.
HALF-LIFE
Discussions here about half-life are mostly courtesy of Bryan in Dutasteride
Forum.
Half-life refers to the time (usually in hours) required for one-half the dose
of a drug to be excreted from the body. The following half-life information
about Avodart™ or dutasteride is found in the prescribing information:
"The terminal half-life of dutasteride is approximately 5 weeks at steady state.
The average steady-state serum dutasteride concentration was 40 ng/mL following
0.5 mg/day for 1 year. Following daily dosing, dutasteride serum concentrations
achieve 65% of steady-state concentration after 1 month and approximately 90%
after 3 months. Due to the long half-life of dutasteride, serum concentrations
remain detectable (greater than 0.1 ng/mL) for up to 4 to 6 months after
discontinuation of treatment. "
Avodart or Dutasteride has relatively much longer half-life than Propecia.
However, it does not necessarily mean that you can reap substantial cost savings
because in the long run, you have to sustain the same amount of medication in
your body in order to see results. You might be able to "defer" the money you
spent on Avodart, but not cutting the overall amount you spend. The real
significance of long half-life is that it allows for very flexible dosing
schemes. You can take a small amount daily or a larger dose less frequently. But
the key is to average the same amount. For example, if your goal is to average
0.5mg in your body, then you can take 0.5mg daily or 3.5mg weekly.
Note that while Avodart or Dutasteride has a long half-life, it does not
necessarily mean that the medication will simply stay fixed at the highest "peak"
level all the time.
DR. LEE'S RESPONSE RE: PRESCRIBING AVODART FOR HAIR LOSS
October 10, 2002 post - This is from HairSite's Dutasteride forum. Note that we
have not verified if the email is indeed from Dr. Richard Lee at Regrowth.
AVAILABILITY & PRICE
Please read the anchored topic in the Avodart forum, availability and pricing is
contantly updated there.
GETTING A SCRIPT
Someone has come across www.medicalwellnesscenter.com which will write us a
script for about $50. Many have already tried their service. The beauty of this
service is that we are NOT obligated to purchase Avodart from any pharmacy. We
can choose to fill the script at ANY pharmacy of our choice. This site is
legitimate and if you want to read about posters' comments about this website's
service, please go to Avodart forum 35. Non U.S residents can take advantage of
their service as well.
AVODART / DUTASTERIDE FORUM
--------------------------------------------------------------------------------
AVODART™ IS INTENDED FOR THE TREATMENT OF BENIGN PROSTATE ENLARGEMENT. PLEASE
CHECK WITH YOUR DOCTOR IF HE WILL PRESCRIBE AVODART™ AS A HAIR LOSS TREATMENT.
AVODART™ is a registered trademark of GlaxoSmithKline.
9.)
WHAT IS DUTASTERIDE (AVODART™ AVOLVE™) /Nueva medicina para la caida del cabello
!!!
Source:
http://www.dutasteridedirect.com/
Dutasteride (Avodart, Avolve) - a new, emerging treatment for hair loss
DutasterideDIRECT.com is your online source for news and information on the
latest treatments for hair loss. This FAQ has been compiled from a number of
sources to give an overview of the use and effects of Dutasteride.
What is Dutasteride (Avodart, Avolve)?
Avolve is the European brand name for Dutasteride, an oral medicine made by
GlaxoSmithKline for treating symptomatic benign prostatic hyperplasia (BPH) in
men.
The active compound in Avolve, Dutasteride, has the added benefit of treating
genetic male pattern hair loss on the vertex (top of the head) and the mid-scalp
area. Dutasteride is chemically similar to Finasteride, the active compound in
Propecia.
Avolve is also known by its US brand name, Avodart. The medicine has been
approved by the US Food & Drug Administration, and in Europe, for treating BPH.
Avolve is supplied in 0.5mg soft gelatin capsules.
How does Avolve work?
Researchers have discovered that men who suffer from benign prostatic
hyperplasia (BPH), or from male pattern hair loss, have increased levels of the
hormone dihydrotestosterone (DHT).
DHT is produced from testosterone by an enzyme called 5-alpha-reductase.
Biochemical analysis reveals higher levels of 5-alpha-reductase in the
bloodstream of men with BPH, and in the scalps of men with hair loss; and less
of this enzyme in men with no BPH or no hair loss.
Avolve inhibits 5-alpha-reductase, blocking the formation of DHT. This
interrupts a key trigger element in both BPH and development of male pattern
hair loss.
What is dihydrotesterone (DHT)?
DHT is one of several male hormones in the body. DHT is responsible for the
development of the external genitals in the male foetus. However, in adult males
DHT appears to cause:
male pattern hair loss
prostate enlargement
shortening of the growing phase of hair
progressive miniaturisation of hair follicles
decreasing number of visible hairs
acne
How is Avolve different from Propecia?
Both medicines work in a similar way. However, Avolve (Dutasteride) inhibits the
activities of two types of 5-alpha-reductase enzymes. In contrast, Propecia (Finasteride)
only inhibits one type. Avolve has been shown to decreases levels of DHT by 90%
after only two weeks, making it a more powerful and faster-acting weapon against
BPH and hair loss than Finasteride.
What studies and trials have been done concerning Avolve?
A total of 2951 men with moderate to severe BPH were treated with 0.5 mg Avolve
(dutasteride) daily. The study found that acute urinary retention was reduced by
57%, and the risk of benign prostatic hyperplasia-related surgical intervention
was cut by 48% compared with placebo. The drug was shown to be well tolerated.
GlaxoSmithKline also completed Phase II trials for FDA approval of Avolve for
treating hair loss. After six month of treatment, the hair counts measured in a
1 inch diameter circle increased by an average 96 hairs with 0.5mg Avolve daily,
compared to an average 72 hairs with 5mg Propecia (Finasteride) daily.
So these initial trials show that Avolve is around 30% more effective than
Propecia in promoting hair regrowth. However, please note that Avolve has only
been specifically approved for treating BPH. It has not yet been approved
specifically for treating hair loss.
When will Avolve be approved for treating hair loss?
In November 2002 GlaxoSmithKline cancelled its planned Phase 3 trials for Avolve
for treating hair loss. The company has not publicly given a reason for this.
Industry sources speculate that the trials were stopped because the maker thinks
Avolve will be perceived as too similar to Propecia in consumers' minds, and may
not generate sufficient return on investment to justify the cost of the
approvals process as a treatment for hair loss.
However, Avolve is approved by the US FDA and by European bodies for the
treatment of BPH, and so has passed all relevant safety standards.
Will Avolve help hair re-growth for all men?
As with Propecia, Avolve increases the number of scalp hairs, helping to fill-in
thin areas of the scalp. Although results will vary, generally men will not re-grow
all of the hair they have lost. Male pattern hair loss occurs gradually over
time, but Avolve can significantly reduce or delay hair loss. Note that Avolve
is not yet approved for treating hair loss: its effects are a side-effect of its
actions against BPH.
Is Avolve safe?
Clinical trials showed that it was generally well tolerated. Most side effects
were mild or moderate and generally went away while on treatment in both the
Avolve and placebo groups.
Drug-related side effects during the first six months were as follows:
impotence (4.7% vs. 1.7% for placebo)
decreased libido (3% vs. 1.4%)
breast tenderness and breast enlargement (gynecomastia; 0.5% vs. 0.2%)
ejaculation disorders (1.4% vs. 0.5%).
Avolve should not be used in women and children. Women who are pregnant or may
become pregnant should not handle this medicine because of possibility of
absorption and subsequent potential risk to a male foetus.
Men treated with Avolve should not donate blood until at least six months after
their final dose to prevent the medicine going to a pregnant woman through a
blood transfusion.
Men with liver disease should talk to their doctor before taking Avolve.
11.) Synopsis of
Hereditary Poikilodermatous syndromes.
Khalid Al Aboud,M.D
Khalid Al Hawsawi,M.D
Dermatology division,Department of
Medicine,King Faisal Hospital,Taif,Saudi Arabia
Address of corressopndence :Khalid Al
Aboud,M.D,P.O Box 5440
Makkah,Saudi Arabia,Tel 966 2 7382444,Fax
966 2 7384719,e-mail
[email protected]
Aging of the skin is a physiological process.However,there
are a groups of diseases that cause aging of the skin.One of these group is
hereditary poikilodermatous syndromes.It is characterixed by affection of the
skin by ,pigmentary changes(both hypo and hyperpigmentation),atrophy and
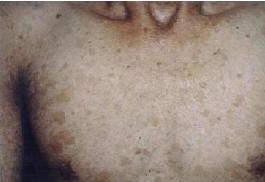
telangectasias(fig .1)
This group include:
·
Rothmund- Thomson Syndrome.
·
Dyskeratosis Congenita.
·
Xeroderma Pigmenosum.
·
Kindler Syndrome.
·
Epidermolysis Bullosa Simplex
Variants.
Table I provide a synopsis for the main
diseases in this group.
TableI:Synopsis of the main hereditary poikilodermatous
syndromes.
|
DISEASE |
INHERITANCE |
ONSET |
SKIN |
HAIR |
NAIL |
TEETH & MUCUS MEMBRANES |
OHTERS |
|
Rothmund Thomson
syndrome (rothmund 1868) |
A.R |
3-6 M ERYTHEMA BLISTERS
ON FACE LIMBS |
POIKILODERMA |
SPARSE |
- |
ABNORMAL DENTITION |
CATARACT SKELETAL
ABNORMAL ALITIES |
|
Xeroderma pigmenosum &
Herbal( Jkaposi 1874) |
A.R |
6M-3Y ERYTHEMA &THEN
FRECKLING |
NEOPLASIA
PHOTOSENSITIVITY |
- |
- |
Oral CA |
EYE &CNS INVOLVMENT |
|
Dyskeratosis Congenita (Zinsser
1906) |
X-Linked recessive.A.D |
5-13 Y NAILS
DYSTROPHILES |
POIKILODERMA (neck) |
- |
+ |
LEUKOKERATOSIS.
à
ANORECTAL & URETHRAL STENOSIS |
HAEMATOL.OGICAL
DISORDERS, NEOPLASIA. |
|
KINDER SYNDROM (KINDLER
1954) |
A.R |
BIRTH: BLISTERS |
PHOTOSENSITIVE WEBBING
OF FINGERS |
- |
+ |
PERIODENTITIS
LEUKOKERATOSIS. ANORECTAL &URETHRAL STENOSIS |
- |
|
WEARY SYNDROME WEAR(JET
AL 1971) |
A.D |
INFANCY:
VESICULOPUSTULES OF HANDS |
ECZEMA (4 M – 5Y)
SCLEROTIC BANDS PUNCTATE PPK |
- |
- |
PERIODENTITIS |
- |
Figure 1:poikilodermatous skin changes in a patient with
Kindler syndrome.
==================================================================
DATA-MEDICOS/DERMAGIC-EXPRESS /AUGUST JOURNAL 2.003/ DR. JOSE
LAPENTA R.
===================================================================
|
