WATER as FUEL - WATERFUEL - WATERCAR - WATERGAS - WATERBURNER - WATER FIRE - WATERPOWER - WATERBREAKER - WATERERA - WATERMOTOR - WATERENERGY -
---------------------------------------------
Home -Introduction -Conclusion -Site Map - Links
Water Electrolysis Systems - Water Explosion Systems - Water Plasma Systems - Other Water Dissociation Systems - Other Systems
---------------------------------------------
#>#># Click here for a LARGER and UPDATED version of this website #<#<#
OTHER WATER DISSOCIATION SYSTEMS :
# - 1982, Francois CORNISH (UK), European patent 0055134A1, 1982, US Patent No. 4,702,894, 1987 Aluminium + water electrolysis. http://members.tripod.com/~anon99/water_engine/index2.html
Water reacted cleverly with aluminium, producing Hydrogen and Aluminium Oxide. Hydrogen is sent to the carburetor. A 900 kg car runs 600km on 20 liters of water and 1 kg of aluminium. Clean energy apart from the process of refining Bauxite into aluminium. The only exhaust product of a hydrogen engine is water!
Patent abstracts : � The present invention is based on the desire of the inventor to be able to provide hydrogen on demand from materials which are in themselves safe to handle� hydrogen is formed by creating an underwater electrical discharge between two electrodes at least one of which is made of a metal as defined above� the metal is aluminium which has the advantage that it is in relatively abundant supply, relatively cheap, is formed with a protective oxide layer on its exposed surfaces and reacts with water at a relatively low temperature. Aluminium wire fed against a rotating aluminium drum has been found to give excellent results to provide hydrogen�
�A convenient way of securing the high voltage required is to employ the conventional distributor and coil arrangement which provides the sparking for an internal combustion engine. Two coils in parallel fed from a common distributor has been found to give excellent results�
In this case hydrogen is fed to a small buffer store and as the pressure in the store exceeds a predetermined level, the electrodes are separated so that hydrogen generation is interrupted. As the pressure drops to a certain level the electrodes are again fed towards one another� the following reaction taking place. 2Al + 3H2O ---> Al2O3 + 3H2
Tested successfully by BMW in 1981 : BMW AG, References: 3895-5538
... Dear Mr. Cornish,
In reply to your telex of 17th October, our findings to date are as follows:
The unit as present assembled in a 2000cc car produced sufficient gas to power the engine continuously. The aluminium consumption averaged out at 180 cm per minute over a 70 minute test run. With the capacitor (as per your specification) connected up, we were able to work in our 14v environment. The water temperature remained low, and even without the radiation system was found to be well between your limits. No acid was found on analysis after the test run.
We however feel that one possible problem area may be the disposal of the oxide deposit. Could you please let us know what your findings have been on this side.
Yours faithfully,
Bayerische Motoren Werke Aktiengesellschaft, Service Division , I.V. Henseler , V. Krause.
--------------------------------------------- Return to the top
#>#># Click here for a LARGER and UPDATED version of this website #<#<#
# - 1990, YOSHIRO NAKAMATSU invented ENEREX a device that enable automobiles to operate on PLAIN WATER. http://community-2.webtv.net/RICHARDPORTER/MADWEBTVSCIENTIST
� at the Monroeville Expo Mart in suburban Pittsburgh, seeking investors and marketers. The start of the 3 days exhibition marked the unveiling of the engine created by YOSHIRO NAKAMATSU, who became known as the "EDISON OF JAPAN" after inventing the computer FLOPPY DISK and the DIGITAL WATCH.
NAKAMATSU said his pollution-free engine, called "ENEREX" runs on tap water alone and can create three time as much power as a standard engine. This information reported in the "STATESMAN JOURNAL" in Salem, Oregon.
The article also stated that as of ay 4, 1990,SO STATED THAT AS OF MAY 4, 1990, MR. NAKAMATSU had more than 2000 patents in the US and Japan.As of 2003 he has over 3000 inventions!
- Dr. Yoshiro Nakamatsu interview by Chic Thompson
Address: http://www.whatagreatidea.com/nakamatsu.htm Changed:12:21 PM on Sunday, February 26, 2006 (Funny how urls about free enegy tend to not work right sometimes, isn't it.)
or http://community-2.webtv.net/RICHARDPORTER2/OneCreativeGeniusAn Changed:7:16 PM on Monday, April 10, 2006
DR. YOSHIRO NAKAMATSU AND HIS "ENEREX" THAT WON THE INTERNATIONAL INVENTORS CONTEST "INVENTOR OF THE YEAR" AWARD IN 1990

Notice how compact ENEREX is. In the TV program Dr. Nakamatau points out some basic principles about ENEREX that are explained in more detail by the "Meyer's Fuel Cell" discussion included on this site.
--------------------------------------------- Return to the top
# - 2005, Amnon Yogev, The Car that Makes its Own Fuel, http://thefraserdomain.typepad.com/energy/hydrogen/index.html
A retired Israeli professor from the Weizmann Institute and one of two founders of Engineuity, has invented a car that produces its own hydrogen fuel from light metals, such as magnesium or aluminum, eliminating all of the infrastructure required for making, transporting and storing hydrogen.
The inventor claims that when the cars become commercial, they will cost about the same as conventional cars and will be emission free.
A coil of the fuel - aluminum or magnesium - is fed into a device called a Metal-Steam combustor that will separate hydrogen out of water that is heated to a very high temperature. The oxygen atoms will bond to the metal forming a metal oxide, freeing the hydrogen. The hydrogen and the steam, which forms when the pressure in the 'fuel' line is reduced below that in the combustor, are sent to the engine where they are used to generate power.
Explanation: The steam will be superheated in the engine and returned to the Metal-Steam combustor to provide the heat needed to generate more hydrogen. The waste metal oxide and remaining unoxidized metal would be removed from the combustor and collected for recycling at the fuel station where the coils of metal are purchased.
A drawback is that the amount of metal needed for fuel, to give the car the same range as a conventional car, weighs about three times as much as the gasoline it replaces. The engine should be a minor modification of ICE's. They claim neither the purchase price or the running cost should be much different than conventional cars. Engineuity plans on developing a prototype in three years if they are able to raise enough money from investors.
I don't believe its that simple. Does it really work? Can some of you bright young readers analyze the cycle and see if it can work? How do you start the car? Do you need a heater for the combustor and where do you get the energy to do that?
Can you produce some excess hydrogen and store it, that sounds expensive. How do you adjust the speed? Somehow you must dump some of the heat produced in the engine I guess or can you throttle the hydrogen and design the combustor to take the high pressure that might result.
Can that be combined with the engine cooling system? What is the response time of the system? You have a volume in the combustor that might have to be heated and cooled to provide response. What about the cost of the Metal-Steam combustor? It must operate at a pretty high pressure and the pressure vessel then adds weight to the car.
I guess there is no exhaust system which saves a bit. I would think the price of the "feed metal" would go up with the price of energy, so there is not a great saving there, if any. I have added some to the cycle description to clarify it, I hope I did it correctly.
IsraCast, The Car That Makes its Own Fuel http://www.isracast.com/tech_news/231005_tech.htm
and http://www.opensourceenergy.org/txtlstvw.aspx?LstID=89df93ed-d9e9-48c5-a725-9feabcfb746f
#>#># Click here for a LARGER and UPDATED version of this website #<#<#
--------------------------------------------- Return to the top
# - AEC Unveils its First Hydrogen Production Demonstration Unit http://www.cleanwatts.com/ http://pesn.com/2005/07/20/9600127_AEC_Demo_Unit_Ready/
Alternate Energy Corporation's H2 1500-A1 demonstration unit produces hydrogen from water, on demand. Production arrangements are being made for shipment later in 2005 at a price projected to be comparable to fossil fuels.
Adapted by Pure Energy Systems News:
BURLINGTON, ONTARIO, CANADA -- Alternate Energy Corporation (AEC) (OTCBB: ARGY) yesterday announced that its first alpha-stage unit, the H2 1500-A1, was recently demonstrated before two separate multinational engine companies in the U.S.
This demonstration was set up to review AEC's small scale, on-demand Hydrogen Production technology and discuss business opportunities. These Demonstration units will power an internal combustion engine and Astris Energi's model E8 2.4 kW Alkaline Fuel Cell for a number of potential commercial customers.
According to their website, "AEC owns a metallurgic formulation which separates hydrogen from water at low cost, requiring no electrical energy or external input, and without utilizing or producing any hazardous waste materials.
AEC's process involves chemical reactions between a proprietary metal alloy mix and the liquid solution. These metals are plentiful, stable in cost and produce effective, highly purified hydrogen utilizing a catalytic process."
Engineers who have reviewed the technology say that this system is capable of producing energy at a price competitive with the current fossil fuel kWh cost of energy.
These demonstration meetings are the beginning of a series of such meetings over the coming months, whereby AEC will be showcasing its hydrogen production technology to a long list of prospective commercial customers, potential licensees, select government and institutional contacts and other interested commercial parties.
An earlier prototype demonstration took place on May 8, 2005 with interested/involved parties at AEC's contracted laboratory in Downsview, Ontario, Canada. Now, this H2 1500-A1, alpha stage unit is designed for portable, on-site demonstrations.
AEC says their company is on an accelerated product development timetable to take advantage of several opportunities with targeted organizations.
Alternate Energy Corporation (AEC) intends to provide a hydrogen fuel system that has mass-deployment economics and provides small-scale, on-demand distributed generation of electricity. Following these product demonstrations with key strategic partners in the first part of 2005, the company plans to then ship initial hydrogen production and electricity generation systems later in 2005.
SOURCE:
- AEC Press Release, July 19, 2005 http://www.cleanwatts.com/news/news.asp?id=76
- AEC Press Release, May 17, 2005 http://www.cleanwatts.com/news/news.asp?id=75
Company website: http://www.cleanwatts.com/
CONTACT: Suzanne Brydon - Investor Relations - Alternate Energy Corporation - (519) 620-2623 -
--------------------------------------------- Return to the top
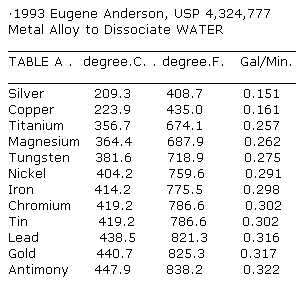
# - 1993, Eugene Anderson,Chemical Reactor Block, Metal Alloy, USP 4,324,777 Material and Method to Dissociate Water at Controlled Rates http://www.rexresearch.com/articles/anderson.htm
� He tinkers with a foot-long, six-inch wide metal cylinder as the group looks on expectantly. He could be a performer or a preacher, knowing just what strings to pull, priming his audience�
The CRB (Chemical Reactor Block), Anderson says, is the product of years of R and D, but more will be needed before they will be able to manufacture and sell it.
He connects one end of a hose to a water faucet, the other end to the hose fitting at the base of the cylinder. It has a hole in its side that allows water to flow through the apparatus. He removes a small wire mesh cage from the top of the cylinder, then holds up a chunk of chalky gray metal. This is the CRB.
He carefully puts the precious substance into the cage. He lowers the cage into the cylinder and turns on the water. As water flows through the fitting, a popping sound is heard.
Casually, Anderson strikes a match and moves it over the mouth of the cylinder. With a whoosh, a flame appears. The beauty of Eugene Anderson's discovery, the real nut of the magical and mysterious CRB material, is that it can supposedly dissociate the H and O of water without suing outside energy and without being consumed in the process.
� Anderson and his uncle [Marion McCoy] saw clearly that the basic problem of the CRB was finding a material that would produce hydrogen without exploding at the same time. Sodium had long been known to liberate hydrogen from water --- but the process was also highly combustible.
Patent abstract :
The preferred catalytic alloy comprises (1) platinum present in an amount of from about 0.7 to about 1.1% by weight, (2) lead present in an amount of from about 42.9 to about 71.5% by weight, (3) antimony present in amount of from about 25.5 to about 42.5% by weight, (4) chromium present in an amount of from about 0.7 to about 1.1% by weight, (5) zirconium present in an amount of from about 4.1 to about 6.8% by weight and gold present in an amount of from about 1.1 to about 1.9% by weight.
A specific example of the alloy comprises about 0.9 wt. % platinum, about 57.3 wt. % lead, about 34.0 wt. % antimony, about 0.9 wt. % chromium, about 5.4 wt. % zirconium and about 1.5 wt. % gold.
�After blending to provide a uniform mixture the resultant mixture is compressed to form a solid mas by application of pressure of about 40,000 pounds per square inch in a graphite mold conforming to the desired shape of the finished product. The compressed mass is disposed in a crucible conforming to its shape and is <>Bheated to an elevated temperature of about 10.degree. C. above the melting point of the mass and this temperature is maintained for about 10.+-.1 minutes. �
�Each reactor block having the extender material identified in Table a is contacted with a fine spray of water at about room temperature in an atmospheric environment. The gaseous effluent from the contact comprising hydrogen and oxygen and burns when subjected to electrical sparking. The volume of gas is dependent upon reactor block surface area and the volume of the water impinging thereon�
#>#># Click here for a LARGER and UPDATED version of this website #<#<#
--------------------------------------------- Return to the top
# - CHEMALLOY Article & Formula - 06/25/01 http://www.keelynet.com/energy/chem.htm
� Chemaloy powderized to about 1,000,000 particles per pound exhibits the same elecritical properties (Fig. 2) as the solid rod. Here it generates slightly more than .5 volt, and in addition decomposes the water, liberating hydrogen�
� This process is further examined in Fig 3. First fill three graduated cylinders with water, one cold, the second warm, and the third hot. Add equal amounts of Chemaloy to each graduated cylinder. Instantly, the graduated cylinder containing hot water liberates hydrogen (Fig. 3A).
Heat is generated by the reaction so that with the passage of a few minutes (Figs. 3B and C) the three graduated cylinders are equally warm and hydrogen production in all three is the same.
One of the most significant uses of powdered Chemaloy may be the warming and loosening of soils that are too cold or compact for optimum seed generation and plant growth. The warming and areation of soil on a laboratory basis is shown in Fig. 4. A sample of dry soil is placed on top of powdered Chemaloy in a glass case. Note the temperature rise from 94 degrees F. to 126 degrees F. Voltage remains approximately at .6.
Chemaloy Smelting Process, from Patent 2,796,345, 1957
Abstracts : In preparing the alloy of the present invention, the following metals and metal alloys are melted together in a crucible in the following proportions to provide the metallic ingredients:
The chemical ingredients are mixed together thoroughly and the acid added and stirred into the dry ingredients until a thin or watery paste-like mass is produced.
...Meanwhile, the metal ingredients in the crucible have been heated until they reach the temperature of approximately 1450� F.... and a layer of fine grain powdered charcoal of approximately a half-inch thickness is deposited on top of the molten metal to form an insulating blanket.
When this charcoal layer has become red in color, the wet mass of chemical ingredients is deposited entirely over the top of the charcoal blanket in a thick layer. Using a suitable pushing device, such as a metal rod, the chemical mass is forced down through the charcoal blanket into the molten metal mixture, a small area at a time. The charcoal blanket shields the remainder of the mass from explosion or excessive reaction.
#>#># Click here for a LARGER and UPDATED version of this website #<#<#
--------------------------------------------- Return to the top
# - 2000, article by Robert Nelson, Water to Gasoline : http://www.rexresearch.com/franch/franch.htm
Wouldn't it be nice if we could burn water for fuel?
Think of all the money we could save, since water costs only 25 cents a gallon this week!
It's a wet dream that has been fulfilled several times. The most recent instance occurred in 1996 at the Indian Institute of Technology (ITT), where 30-years old Ramar Pillai demonstrated the conversion of water to a hydrocarbon fuel by mixing it with a secret herbal formula he had discovered. Scientists were understandably amazed by the experiment, which was organized by ITT chemist N. K. Jha. "It is incredible but true", Jha said.
About two ounces of leaves and bark were boiled in a liter of water, cooled, and a small amount of salt, citric acid, and secret chemicals were added. About a pint of combustible liquid that smells and burns like kerosene was produced within 30 minutes. The National Chemical Laboratory (Pune, India) analyzed the substance and found it to be a pure hydrocarbon with a boiling point of 170� C. The new fuel is more efficient than gasoline, and produces no sulfur exhaust. Researchers at the Indian Institute of Petroleum confirmed the reality of the process.
� In 1916, Louis Enricht announced that he had invented "a substitute for gasoline that can be manufactured for a penny a gallon". As a demonstration, Enricht allowed reporters to inspect the empty gas tank of an automobile. The reporters also tasted the water that Enricht then poured into the tank. He added a green pill, started the car, and gave the reporters a ride around Farmingdale, Long Island. William Haskell, publisher of the Chicago Herald, investigated Enricht's claims. He wrote:
"I examined the entire engine and tank. I even tasted the water before the mysterious green pill was dropped into the tank. Then I opened the petcock and examined the liquid, which now tasted like biter almonds. I also tasted the liquid at the carburator which was the same. I was amazed when the auto started. We drove it around the city without any trouble".
� In 1917, John Andrews approached the US Navy with his claim that he could convert fresh or salt water into a fuel with the same power as gasoline. The chemical costs were about 2 cents/gallon.
Andrews was allowed to demonstrate his invention at the Brooklyn Navy Yard, where a motor boat was fitted with a dynamometer for the test. Commander Earl P. Jessup, who was Captain of the yard, said:
"We gave Andrews a bucket of water drawn from the Navy Yard [fresh water] hydrant by one of the yard attaches. He got into his car with a gallon can which we inspected and found to be empty and a little satchel he carried with him. In about a minute he handed out the filled can which I personally carried to the open fuel tank. While pouring the liquid into the tank, Andrews held a lighted cigarette close to the liquid, which did not ignite. That showed it was not gaseous or inflammable at that part of the demonstration, which to me was most important. The engine caught just as quickly as it would have done with gasoline, and after a moment's adjustment of the carburator, it settled down to its work, developing 75% of its rated horsepower, a remarkable showing with any fuel with so slight a readjustment of the carburator".
In a second test put in an empty room with no possible way to get rid of the bucket of salt water with which he had been supplied, except to empty it into his one-gallon gas can. Commander Jessup said:
"In a minute he emerged with the can filled, and the engine again used it up, no difference being noted between the salt water and fresh. Besides myself, Rear Admiral G.E. Burd, the Industrial Manager of the yard, was present and with the precautions we had taken --- our own Navy engine, tank and carburator and our own men supplying the water --- there was no possibility of deception.
"From a military viewpoint, it is almost impossible to visualize that such an invention means. It is so important that we have hurried an officer to Washington to make a report to the navy Department. It is obvious that Andrews has discovered a combination of chemicals which breaks down water to a form that is inert until mechanically vaporized by the carburator, when the spark causes it to burn as gasoline burns".
� The next person to demonstrate the conversion of water to fuel was Guido Franch, a former coal miner who tried for nearly 50 years to find financiers for his product. He too used a green powder to turn water into 105-octane fuel. He called it "Mota", which is atom spelled backwards.
Franch demonstrated Mota hundreds of times, but never produced it commercially. He did, however, sell about 3000% of his rights to interested investors. In 1973, Franch was subpoenaed to appear in Chicago's Federal Circuit Court "with any records relating to the purchase or the proposed purchase of any fuel, fuel powder, or fuel formula in your possession". He demonstrated his Mota transmutation in the presence of judges William Bauer and Philip Romiti, who believed what they saw, and Franch was acquitted of charges of fraud.
The fuel is produced with one pound of the reagent in 50 gallons of water. It burns clean and leaves no residue. In one demonstration with a lawnmower, it ran for about 15 minutes on a small amount of Mota-treated water. An equal amount of gasoline lasted only 3 minutes. Mota fuel is very sensitive to sunlight, which will turn it back to water with a white powder residue.
� "The granules are dark olive green. As they enter water, they dissolve in a string of green, which begins to spread fiber-like throughout the water. As the water begins to react, there is a swirling effect. Reaction is complete in a few minutes. If the crystals are mixed in 1:1 ratio with water, the resulting fluid is highly explosive and can be detonated by a small shock. But it isn't shock-sensitive when mixed at a normal ratio of one ounce of powder per half gallon of water. The finished fuel is lighter than water".
--------------------------------------------- Return to the top
# Other Catalytic concepts:

--------------------------------------------- Return to the top
#>#># Click here for a LARGER and UPDATED version of this website #<#<#
# - W. Gunnerman, Engines that run on water? http://www.himacresearch.com/docs/gunn2.htm
By Otis Port, Business week, 08/09/94, 20% Water + 5% Emulsifier + 80% oil
Rudolf W. Gunnerman has a tiger by the tail--the Exxon tiger. If the technology that the 66-year-old inventor has spent $6 million and the past seven years developing lives up to his claims, cars and trucks could one day be running on a fraction of the gasoline and diesel fuel they now use. Ditto for buses, planes, trains, and anything else powered by an internal-combustion engine--from lawn mowers to huge electrical generators.
Gunnerman claims to have a technology that enables engines to burn a mixture of half fuel, half water. Yes, water. What's more, he says, the mixture gets 40% better mileage from the gasoline it contains and emits significantly less pollution because engines run cooler. In particular, tailpipes emit virtually no nitrogen oxides --the principal source of smog.
Sound crazy? Maybe, but Caterpillar Inc. is so intrigued that in early July it formed a joint venture with A-55 LP, Gunnerman's tiny, nine- person company in Reno, Nev. A-55 is short for aqueous 55%, the amount of water by weight in the patented fuels. But the key ingredient is 0.5% of a secret emulsifier that enables fuel and water to mix--and stay mixed. Gunnerman financed his work with royalties from other patents, especially th ose covering the making of pellets for woodstoves.
� Gunnerman believes the water gets broken down into hydrogen and oxygen, and the hydrogen contributes energy to the combustion process. That's because there is one additional trick in h is patented process: A small piece of nickel must be attached to the crown of each piston or the top of the cylinder heads. The nickel seems to act as a catalyst in `dissociating' the water, says Gunnerman.
Hogwash, says David B. Kittelson, a mechanical engineering professor at the University of Minnesota. Researchers have been burning 50%- water fuels since the 1940s, and "there's this myth that you're burning the water.'' Actually, he says, the water j ust allows the engine to run hotter and not melt. World War II bombers wouldn't have been able to get off the runway without their water-injection turbochargers.
� In Minnesota, Gregory Felt, chief operations engineer for the state' s Transportation Dept., admits he was "the biggest skeptic around". So he asked Gunnerman's team for a live demo: Mix up a fresh batch with local tap water and diesel fuel. When the blend was used to fire up a model 453 engine from Detroit Diesel Inc., "it had the cleanest exhaust I've ever seen coming out of a diesel", says Felt. "If it really does what it seems, this is big.'' How big? "If this proves out, it could reduce the U.S. trade deficit by almost half, by eliminating the need to import oil,'' says John D. Peters, who tracks emerging transportation technology for Minnesota.
� Gunnerman's next project: a fuel that would eliminate gasoline altogether. He calls it "X fuel.'' It's a mix of naphtha and water. Naphtha comes out of the process of refining oil earlier than gasoline, so it costs 50% less. It 's enough to send the Exxon tiger into a tizzy.
About Gunnerman See also http://detnews.com/menu/stories/35563.htm
--------------------------------------------- Return to the top
# - POWERBALLS, Powerball Technologies Inc.
A company in Utah has come up with the idea of compressing sodium hydride powder into balls and then coating these ping-pong balls with plastic so that they can be easily and very safely transported. Now if you drop one of these balls in water, nothing will happen. But if you break one open your get tons of H2 gas at high pressure if in a sealed container.
They have designed a pressure reactor that contains water and a bunch of powerballs floating around. Inside the reactor chamber is a device that captures and then slices a powerball thereby releasing the NaH into the water. The cutting device is controlled by a pressure switch on the gas feed line, thus when gas pressure drops it triggers another ball to be cut, pressure then goes back up. This gives you hydrogen-on-demand (HOD) and a pressurized buffer supply.
Is this enough to run an internal combustion engine? Yes. But it would be more efficient to use this hydrogen gas in a fuel cell and run an electric car.
In either case, this technology approach will create a closed loop economic recycling system whereby gas stations would now sell powerballs and dump into storage the sodium hydroxide (NaOH) and plastic casings to be completely recycled back into more powerballs. No waste. This creates a new economic industry that does NOT pollute the environment.
Can the reactor explode? No. Since the powerballs are floating in water and only enough gas is produced at any one time to maintain an operating pressure level that is well under the operating strength of the pressure vessel, there is no chance for a pressure explosion. Any hydrogen gas leaks would also not explode, as this requires the hydrogen to mix with atmospheric oxygen in a 2:1 mix before it would become explosive. Any greater or lesser mix and the hydrogen just burns as a clear flame.
Even if the reactor tank is punctured in a collision only a small amount of gas would be released and the remaining balls would just be left floating in water. Only upon very high temperatures would the water in the reactor turn to steam and at this point pressure relief valves would open to let gas and steam out. And when all the water is boiled off, then the plastic shells have to melt releasing the NaH, which will have nothing to react with by that time. Safe? Yes
--------------------------------------------- Return to the top
# - 1998, New Hydrogen-Producing Reaction Could Lead to Micropower Sources, http://www.ornl.gov/info/ornlreview/v33_2_00/micropower.htm
See also related article: http://www.ornl.gov/info/ornlreview/v33_2_00/hydrogen.htm
A new method for the sustained production of hydrogen has been discovered by researchers in ORNL's Chemical Technology Division (CTD).
The discovery could lead to the development of palm-sized fuel cells that cost only a few cents a piece. The fuel cells could be used to power compact environmental sensors for the U.S. military, as well as cell phones, cameras, and portable audio and video equipment.
Soldiers could easily carry these fuel cells on the battlefield and recharge them by adding iron powder and vinegar and then shaking them. These cells could serve as micropower sources for sensors that can detect the presence of hazardous gases and emissions from nearby chemical and biological warfare weapons.
In the summer of 1998, CTD's Jonathan Woodward and researchers John Getty and Mark Orr tried a new way to make hydrogen from sugar, which involved the deposition of the metal platinum on a glucose-digesting enzyme. The experiment worked.
"After several different experiments," Woodward says, "we then observed that mixing iron powder with water also produced hydrogen at ambient temperatures, but the production was not sustained. Then we discovered that if we add gluconic acid as well as iron powder to the water, we obtained sustained hydrogen production under certain conditions."
Gluconic acid is an organic acid consisting of carbon, hydrogen, and oxygen (C6H11O7) that is produced from glucose sugar, an abundant and renewable carbon source. Woodward noted that the sustained hydrogen-production reaction works well under three conditions: a temperature of 80�C, neutral pH, and the absence of oxygen.
Although the mechanism of the reaction is not fully understood, Woodward says that iron may be serving as the active catalyst for the production of hydrogen gas from water under anaerobic conditions. During the reaction, the metal iron (Fe) is converted to an iron-oxide compound called magnetite (Fe3O4). The magnetite would then be reduced back to iron in the oxygen-free atmosphere containing gluconic acid. Thus, the iron catalyst would be regenerated from the magnetite, enabling the continuing production of hydrogen.
"We found that after 100 hours of the experiment, we lost little metal and got more hydrogen than we expected," Woodward says. "We generated more hydrogen than the typical metal displacement reaction where iron is normally consumed. We believe that some of the hydrogen is produced by the reaction of the iron metal with the organic acid, but more experiments must be done to prove that.
"Hydrogen produced this way could be used as a power source for fuel cells that power sensors and cameras requiring very low current in the micro- to milliampere range. Larger-scale applications may also be possible."
#>#># Click here for a LARGER and UPDATED version of this website #<#<#
--------------------------------------------- Return to the top
# - Eli Greenbaum, Biological water splitting. http://www.ornl.gov/info/ornlreview/v33_2_00/hydrogen.htm
Eli Greenbaum studies algae being used to produce hydrogen from water in an illuminated flask.
Hydrogen is commonly stripped from natural gas, but that process leaves carbon dioxide, which must be disposed of in an environmentally acceptable way. The conventional way to produce hydrogen without generating carbon dioxide is to separate hydrogen from oxygen in water using electrolysis. � Today's electrolytically produced hydrogen costs around $30 per million British thermal units (Btu); by comparison, natural gas costs about $3 per million Btu, and gasoline costs about $9 per million Btu�
� Water molecules can be split into hydrogen and oxygen atoms using algae, one-celled organisms that thrive in water. ORNL researchers Eli Greenbaum (an ORNL corporate fellow), James Lee, and Steve Blankinship-all in the Chemical Technology Division (CTD)-have discovered that the green alga Chlamydomonas reinhardtii can produce hydrogen and oxygen from water under certain conditions. "It's the biological version of electrolysis," Greenbaum says. "The goal of the research is to replace conventional electrolysis with a renewable biological process for hydrogen production."
These algae normally grow new cells by photosynthesis, using carbon dioxide from the air in the presence of sunlight. But after placing the aquatic organisms in a large flask of water illuminated by lamps, the ORNL researchers "trick" the algae by depriving them of carbon dioxide and oxygen. As a result, a normally dormant gene becomes activated, leading to the synthesis of the enzyme hydrogenase. �
� Membrane separation technologies are being developed that will separate the hydrogen from the oxygen more efficiently. Because the algal hydrogenase eventually shuts down from exposure to oxygen, Michael Seibert and Maria Ghirardi, researchers at the National Renewable Energy Laboratory, are working to create a mutant organism that makes a hydrogen-producing enzyme that is less sensitive to oxygen. The third challenge is to optimize the ability of the algae to use light.�
"Eventually," Greenbaum says, "we hope to have mutant algae that will produce 10 times more hydrogen if we increase the light intensity 10 times."
Jonathan Woodward and his associates in CTD are trying to use enzymes to make hydrogen from the cellulose present in old newspapers, grass clippings, and other waste products of renewable resources. The first step is to transform cellulose into glucose sugar, and the second step is to convert the glucose product and its byproduct, gluconic acid, into hydrogen. The second step has proved easier.
In 1996 Woodward and his colleagues reported an important advance. They learned how to produce a molecule of hydrogen from a molecule of glucose using two enzymes (called extremozymes) produced by microorganisms that grow under extreme temperatures.
In October 1999, CTD researchers Woodward, Mark Orr, Kimberley Cordray, and Greenbaum reported producing 11.6 hydrogen molecules for every glucose molecule in the substrate. The researchers achieved 97% of the maximum stoichiometric yield possible-12 hydrogen molecules for each glucose molecule. This is the highest yield of hydrogen ever obtained from glucose by a biological process. The results are to be published in an upcoming issue of Nature.
This high stoichiometric yield of hydrogen from glucose was attained through an "oxidative pentose phosphate cycle" using 11 enzymes. In this cycle, glucose is oxidized completely to the compound NADPH and carbon dioxide. In the presence of the extremozyme hydrogenase, hydrogen is released. This extremozyme produced by the bacterium Pyrococcus furiosus is also one of only two such enzymes known to accept electrons from NADPH to produce hydrogen.
--------------------------------------------- Return to the top
#>#># Click here for a LARGER and UPDATED version of this website #<#<#
# - 1974, Eric COTTEL, UK, Originally printed in "Newsweek", June 17, 1974.
In the wake of the energy-crisis a 50-year-old British-born inventor named Eric Cottell �
Cottell reasoned that if water could largely replace air as a source of oxygen in combustion, this would avoid the large amounts of nitrogen introduced by the air and thus eliminate much of the noxious nitrogen oxides.
"To accomplish this, he turned to a device he had patented 22 years ago an ultrasonic reactor that emulsifies heavy liquids and is widely used today to prepare such products as Worcestershire sauce, ketchup, cosmetics and paint.
By refining the reactor, Cottell was able to break water into particles about one fifty-thousandth of an inch in diameter and to disperse them evenly in oil (or gasoline) to create an emulsion that was 70 percent oil and 30 percent water. When this emulsion was burned, Cottell found :
... (1) that there were far fewer waste products and
... (2) that the small water droplets expand on heating, then explode into steam, in turn shattering the oil into finer particles, and thus increasing the surface area of the fuel exposed for burning.

"Last month Cottell divided his time between Washington, in talks with officials of the Federal Energy Office, and Detroit, where he consulted with engineers working to meet the tight 1976 automobile emission requirements.
So far, auto tests have shown that with an ultrasonic reactor attached to a carburetor, a car can get almost DOUBLE the normal miles per gallon of gasoline with negligible exhausts.
Cottell's company, Tymponic Corp. of Long Island, N.Y., is also about to produce units for home oil burners that will be no larger than a flashlight and cost $100 to $150.
"Last winter, two Long Island schools converted to Cottell's system, and both reduced their fuel usage by about 25%. Adelphi University reports that it SAVED more than 3,500 gallons of oil per week! and REDUCED soot output by 98 PERCENT."
This file points to a possibly useful technique for those working with water dissociation for the purpose of hydrogen fueled motors. The smaller the particle is, the less energy required to dissociate into consecutively smaller units. This is probably one of the inspirational sources used by Stan Meyers for his "fractioning" process.
Ultrasonic generators can be both mechanical or electronic in nature. Transducers can be easily purchased with resonant frequencies ranging from 20KHZ to 40KHZ.
--------------------------------------------- Return to the top
# Other Systems to break the water Molecule :

# Some systems using Light for water dissociation :

# Using Magnetic Forces to split water :
# Some long time known systems for water dissociation :
# Some ideas about thermal dissociation :
- Or using HYDROXY Gas to heat a tungsten element to around 2,900 oC (if it can stay stable), with very hot water vapor coming from a Vortex Room that will break in contact with this very hot tungsten element.
Need electricity to start to produce Hydroxy and water vapor, but immediatly after the vapor start to break, we can use a part of the produced gas (if it is also Hyroxy Gas) to heat the tungsten element and produce the vapor, under an over-unity process; as we now that Hydroxy gas can give 3 to 4 times more energy that it needs to be produced.
--------------------------------------------- Return to the top
# - 2005, The Kentucky Water Fuel Museum at Lexington KY ; http://waterfuelmuseum.com/
1805-2005: 200 years of turning water into fuel!
It has been over 200 years since humans first turned water into hydrogen and oxygen, using electric current. In 1805 came the first car with an internal combustion engine that ran on water!
What happened next? The purpose of the Water Fuel Museum is to tell the interesting tale of water fuel technology development. In 1875 Jules Verne wrote in "Mysterious Island" that 'water is the coal of the future'. He was no prophet; it was already happening! Patent after patent has been filed in the U.S. and abroad attesting to the reality of this awesome and non-polluting fuel source! It could even reverse global warming by producing oxygen in excess of what is needed for combustion!
Shouldn't people know about this? We think so. That's why we're opening the world's first museum dedicated to telling the story.
Come see us on opening day: October 1, 2005 at 1012 Manchester Street, behind Rupp Arena. Hours:10:00 to 6:00 Monday thru Saturday. Adults $4.50 Seniors (60+) and children $3.50 Y'all come!
--------------------------------------------- Return to the top
- Some General Information :

note: water from air is a main goal to free energy supply, because harvesting the humidity of air to provide drinking water to everybody is possible AND very important. 3 days without drinking and u die.
--------------------------------------------- Return to the top
---------------------------------------------
Home -Introduction -Conclusion -Site Map - Links
Water Electrolysis Systems - Water Explosion Systems - Water Plasma Systems - Other Water Dissociation Systems - Other Systems