Nuclear Decay: What it is, what it does?
Unstable nuclei, called radioactive isotopes, will undergo nuclear decay to
make it more stable. There are only certain types of nuclear decay which means
that most isotopes can't jump directly from being unstable to being stable. It
often takes several decays to eventually become a stable nucleus. When unstable
nuclei decay, the reactions generally involve the emission of a particle and or
energy. Half-lives are characteristic properties of the various unstable atomic nuclei and the particular way in which they decay. Alpha and beta decay are generally slower processes than gamma decay. Half-lives for beta decay range upward from 10-2 sec and, for alpha decay, upward from about 10-6 sec.
Bismuth-209 has the longest half-life of 2x1019 years. Half-lives for
gamma decay may be too short to measure (~ 10-14 second), though a
wide range of half-lives for gamma emission has been reported.
There are five types of nuclear decay:
 |
Alpha Decay - The strong force, despite its strength, has a very
short range; it can't even reach from one end of a fair-sized atomic
nucleus to the other. If a proton is at the edge of a big nucleus, it
can feel the pulling strong force only from the particles in the
neighborhood, but there is an electromagnetic force, which tends to push
it out, all the way from the other side of the nucleus. There is a
sensitive balance between these two competing forces. The nucleus needs
not to acquire extra energy to escape; the quantum mechanical effect
called tunneling allows a certain probability of escape through a
potential wall. Alpha decay, in which just a small chunk breaks off from
the main nucleus, is a rather mild case of fission; in more dramatic
examples, the nucleus can break more or less in half. The broken-off
chunk most often is packed into a helium nucleus (alpha particle)
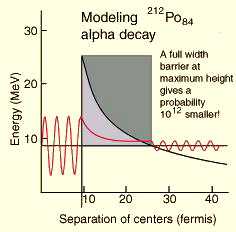
because it is in a more stable form. Figure 1 shows the effect of
tunneling through the Coulomb barrier; the nucleus has a small
probability of escape to the outside depending on the height and width
of the wall. |
Figure 1. Alpha Decay
|
|
 |
Beta Decay - The weak interaction is responsible for the instability
of "free" neutrons, which decay according to the reaction: n ==> p +
e + electron-anti-neutrino with a lifetime about 15 minutes in a process
known as beta decay. Neutrons in a nucleus are subject to the protection
of the nuclear and the electromagnetic forces from the other nucleons,
and they will remain stable provided there are not too many of them. If
there are too many, such protection would not be sufficient for all of
them to remain stable, and the nucleus would undergo beta decay. Figure
2 shows that in the beta decay process, the down quark
turns into an up quark (thus changes the neutron to proton) by emitting
a W- meson, which decays into an electron and an
electron-anti-neutrino. |
Figure 2. Beta Decay
|
|
 |
Gamma Decay - In gamma decay, a nucleus changes from a higher energy
state to a lower energy state through the emission of electromagnetic
radiation. It happens usually after the transmutation of the nucleus;
the end product has to re-arrange the occupancy of energy levels in
order to arrive at a more stable state. The number of protons (and
neutrons) in the nucleus does not change in this process, so the parent
and daughter atoms are the same chemical element. In the gamma decay of
a nucleus, the emitted photon and recoiling nucleus each have a
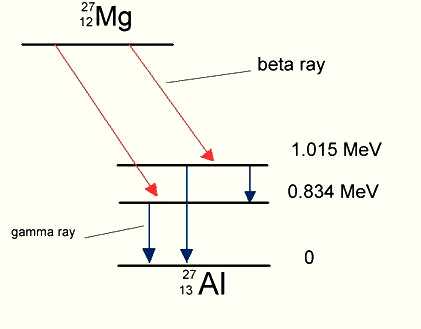
well-defined energy after the decay. Figure 3 shows the adjustment
of energy level by emitting gamma ray after Mg has transmuted into Al.
|
Figure 3. Gamma Decay
|
|
Positron Emission - Positron emission is a type of beta decay, referred to
as "beta plus"(ß+ ) or inverse beta decay. In beta plus decay, a proton is
converted to a neutron, a positron and a neutrino via the weak interaction.
This spontaneous nuclear process releases an amount of energy equal to the
energy equivalent of the rest mass that disappears in the process. The
positron and neutrino are created in the nucleus at the moment of
disintegration. The "endothermic reactions" receives the energy from the
nuclear fission. That positron decay is a nuclear process is consistent with
the fact that the decay of free protons by positron emission is not observed
in nature. On the other hand, the beta decay of free neutrons is a familiar
fact. The positron is made of anti-matter and does not last very long in the
world of ordinary matter. As soon as the positron meets an ordinary electron
the two particles annihilate and the energy contained in their mass appears as two
gamma-rays of 0.5 MeV each, flying off in opposite directions.
 |
Positron
radioactivity is therefore always accompanied by the emission of gamma
rays with an energy of about 0.5 MeV in addition to any other gamma-rays
which might be emitted. Example isotopes, which emit positrons are C-11,
N-13, O-15 and F-18. These isotopes are used in positron emission
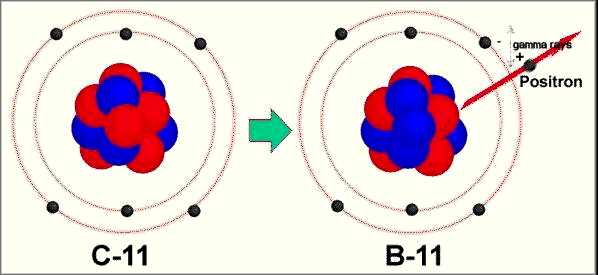
tomography (PET). Figure 4 shows the transmutation of C-11 into
B-11 by positron emission. |
Figure 4. Positron Emission
|
|
Electron Capture - Electron capture is a decay mode for nucleus that will
occur when there are too many protons in the nucleus of an atom, and there
isn't enough energy to emit a positron. In this case, one of the orbital
electrons is captured by a proton in the nucleus, forming a neutron and a
neutrino. Since the proton is essentially changed to a neutron, the number of neutrons increases by 1, the number of protons
decreases by 1, and the atomic mass remains unchanged.
 |
By changing the
number of protons, electron capture transforms the nucleus into a new
element. Electron capture is also called K-capture since the captured
electron usually comes from the atom's K-shell. Figure 5 shows
another way of transmuting C-11 into B-11 by electron capture. |
Figure 5. Electron Capture
|
|
Table 1 below summarizes
the various types of nuclear decay with a few examples.
| Type |
Emission |
Penetrating Power |
Example |
| Alpha Decay |
Helium nuclei |
1, stopped by skin, very damaging due to ionization |
92U238 =>
90Th234 + 2He4
Applicable
to nuclei with Z>83, see Figure 14-02 |
| Beta Decay |
Electron, high speed |
100, penetrates human tissue to ~ 1 cm |
53I131 =>
54Xe131 + -1e0
Applicable
to nuclei with high neutron-proton ratio |
| Gamma Decay |
Photons, high energy |
10000, highly penetrating but not very ionizing |
92U238 =>
90Th234 + 2He4 + 2 photon
Energy lost from settling within the nucleus after transmutation |
| Positron Emission |
Positron |
100 |
6C11 =>
5B11 + 1e0
Applicable to
nuclei with a low neutron-proton ratio |
| Electron Capture |
Electron, inner shell |
Neutrino |
37Rb81 + -1e0
=> 36Kr81 + neutrino
Applicable to nuclei
with a low neutron-proton ratio |
Table 1. Types of Nuclear Decay
go to top